Ophthalmic gel

Eye drops are commonly used in ophthalmology, but most of the drug is lost before it takes effect, and bioavailability is often less than 5%!
To solve this problem, in situ gels were developed - they are liquid in the bottle and are rapidly converted into a non-chemically crosslinked semi-solid gel when dropped into the eye to achieve a slow-release, long-lasting effect and improved bioavailability.
Get Free Quote
Experimental parameters
| Dissolution Apparatus | RT700 |
| Cell | Large Cell (22.6mm) |
Experimental result
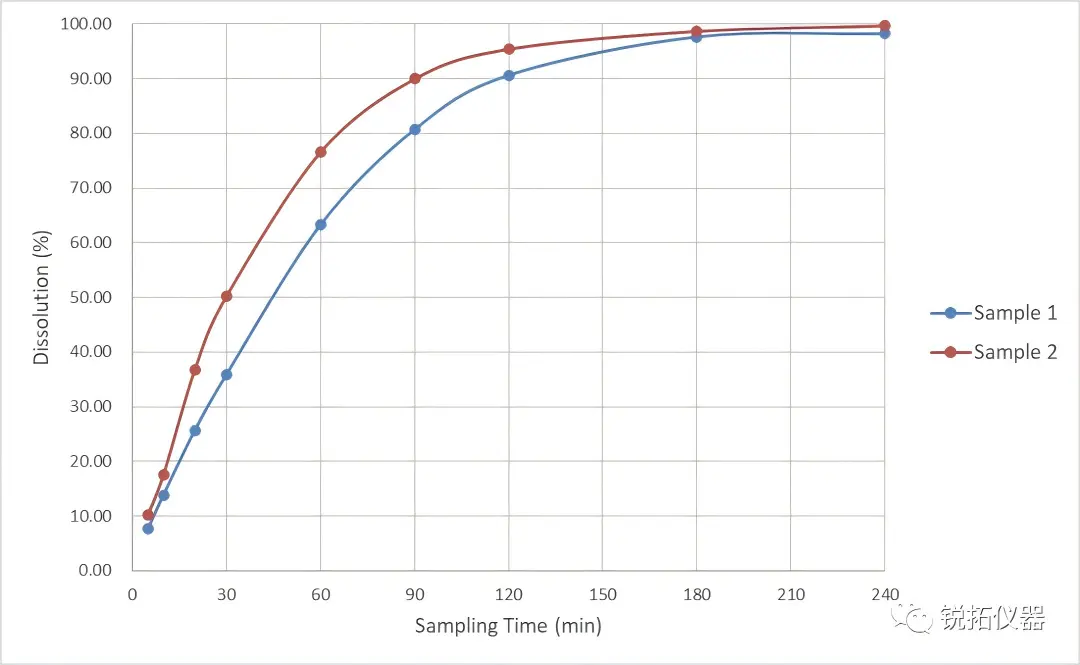
Case Sharing: Effortlessly distinguish differences in prescription processes We tested two different manufacturing process batches (Sample 1 and Sample 2) of an ophthalmic gel.
Consistent and reliable results: The relative standard deviations of the final dissolution rates of the two batches are only 0.66% and 0.88% respectively, which is extremely reproducible!
Strong differentiation ability: The test results clearly show that the release rate of Sample 2 is significantly faster than that of Sample 1.
This proves that the Raytor RT7 Flow-Through Cell Dissolution System not only stabilizes the release process of ophthalmic gels, but also effectively distinguishes the subtle differences between batches of different production processes, providing a reliable basis for quality control and process improvement!
Flow-through cell method allows us to get rid of the constraints of traditional methods and bring the in vitro release determination of special dosage forms closer to the expectations of drug developers!

