BCS Class Ⅳ drugs
BCS Class Ⅳ drugs refer to a class of drugs in the Biopharmaceutics Classification System (BCS) that are primarily characterized by poor solubility and low permeability. This means that they dissolve poorly in media and have difficulty in crossing the intestinal wall to be absorbed by the body, resulting in low bioavailability.
Get Free Quote
Experimental parameters
| Sampling | Peff | Papp | Similarity to the original study |
| original research drug | -5.818 | 1.52 | 100.0%(standard of reference) |
| Products of Manufacturer A | -5.791 | 1.62 | **106.6%** |
| qualified batch of Manufacturer B | -5.767 | 1.71 | **112.5%** |
| regular batch of Manufacturer B | -5.833 | 1.47 | **96.7%** |
Experimental result
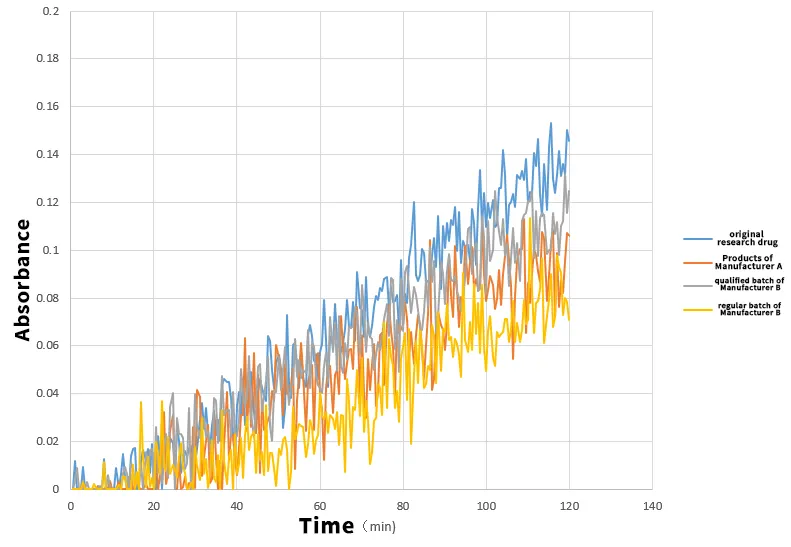
Solution: Using the Raytor NCE DP, key permeability data were determined by the PAMPA method to simulate the absorption characteristics of drugs in the human small intestine (pH 6.5 environment). 1. Permeability similarity assessment -Desired goal: The closer the Papp value is to the original's 1.52, the higher the similarity is - Result Analysis: - qualified batch of Manufacturer B: Papp of 1.71, +12.5% deviation from the original study - products of Manufacturer A: Papp of 1.62, +6.6% deviation from the original study - regular batch of Manufacturer B: Papp is 1.47, deviation from original research -3.3% 2. Interpretation of the consistency evaluation perspective - regular batch of Manufacturer B: closest to the original study in permeability (difference of only -3.3%), reflecting better quality consistency - products of Manufacturer A: permeability is slightly higher than the original study, although within the BE standard, there are some differences with the original study - qualified batch of Manufacturer B: permeability is significantly higher than the original study, although it passed the BE, but it is the most different from the original study in terms of permeability characteristics
Conclusion.
Qualified batch of manufacturer B that has passed the consistency evaluation, although meeting the clinical BE test standards, has significant differences in in vitro permeability characteristics compared with the original research (+12.5%).
This suggests that we:
- The BE test has proved that its safety and effectiveness meet the standards, but the differences in permeability characteristics deserve attention.
- regular batch of Manufacturer B was closer to the original in permeability profile instead.
- There are permeability fluctuations between batches of different processes, which need to be strengthened to control the consistency between batches.
Demonstrates the unique value of Raytor NCE DP system in bioequivalence studies of chemical generics:
- ❶ Accurately quantify similarity: provide objective data to accurately assess the consistency of generic drugs with the originator in terms of key quality attributes
- ❷ Reveal potential differences: Even products that pass BE can be found to be different from the original in terms of underlying characteristics
- ❸ Monitor batch fluctuations: help identify quality fluctuations between batches of products from the same company
- ❹ Assist in process optimization: Provide a clear direction for companies to optimize their production processes and pursue a higher degree of similarity with the original .

