Semaglutide
Semaglutide is a novel long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA), which is structurally similar to natural human glucagon-like peptide-1 (GLP-1), with up to 94% amino acid sequence homology. and it is used for blood glucose control in adult patients with type 2 diabetes mellitus.
Get Free Quote
Experimental parameters
| Experimental condition | Control experiment | Test | Reference |
| Bionic membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane |
| Supply side | pH 6.5 buffer | pH 6.5 buffer | pH 6.5 buffer |
| Receive side | pH 7.4 buffer | pH 7.4 buffer | pH 7.4 buffer |
| API | Semaglutide | Semaglutide | Semaglutide |
| Excipient | No excipients added | Contains a penetration enhancer | Novo Nordisk Original Research Excipients |
Experimental result
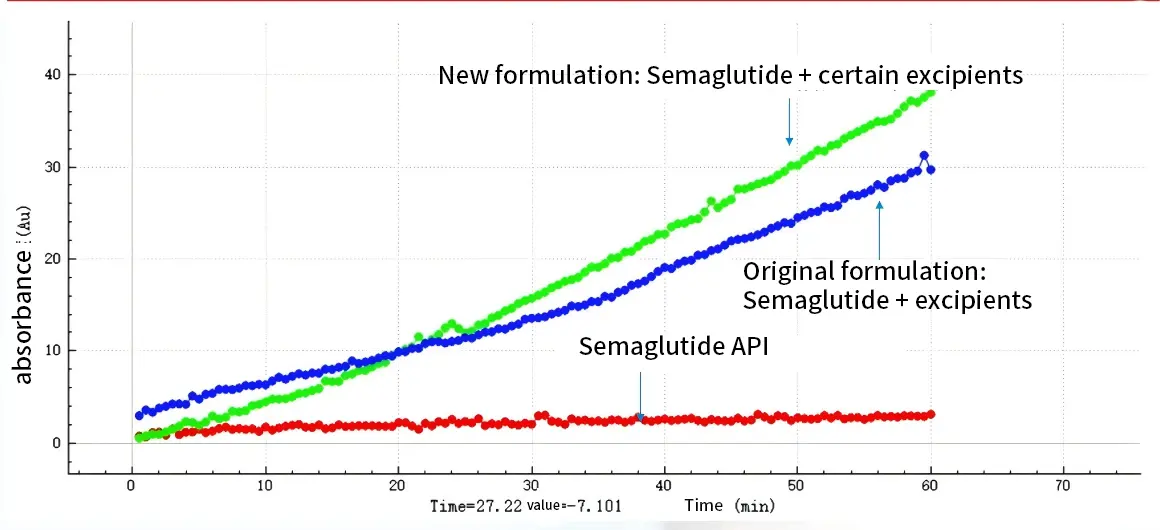
The permeability model in Raytor NCE DP was used to study the compatibility permeability of excipients & api (with PAMPA method) Reference: The reference of semaglutide is a new generation GLP-1 (glucagon-like peptide-1) analog developed by Novo Nordisk. Test sample: The addition of other excipient is used for permeability comparison with the reference.
Conclusion:
The results of the PAMPA experiments in the NCE DP suggest that semaglutide with some kind of permeation enhancer can significantly enhance its absorption (much greater permeation than a pure API) and perhaps have a better absorption enhancement effect than the original formulation.
Discussion:
Peptide drugs are difficult to make into oral peptide drugs because of their poor absorption ability. but if you can find suitable excipients to enhance their permeability, the peptide drugs made into innovative formulations are also very valuable research and development programs. With NCE DP's PAMPA permeation model, the permeation ability of various excipients with peptide drugs can be studied in a high-throughput and high reproducibility, making your peptide drug prescription research easier.

