Synthetic membranes

In drug research, in vitro and in vivo correlation (IVIVC) is a predictive model that can predict the in vivo performance of a drug based on its in vitro performance. For the IVIVC of semisolid preparations, in addition to the in vitro release test (IVRT), the in vitro penetration test (IVPT) also needs to be considered.
The FDA recommends using removed human skin to build an in vitro model for IVPT, but due to the variability of human skin and related ethical issues, researchers are seeking alternatives to human skin. At present, these substitutes are animal skin (such as miniature pigs, dentures, etc.) and synthetic membranes (made of cellulose, polymers, etc.), among which hydrophobic synthetic membranes have been found to be applicable to the pseudo-keratin layer. Therefore, IVPT conditions were constructed by selecting synthetic membranes of different materials and media of different concentrations to determine whether the synthetic membranes had the potential to perform IVPT and predict the performance of drugs in vivo.
Get Free Quote
Experimental parameters
| Test system | Raytor RT800 Automated Transdermal Diffusion System |
| Diffusion cell | The Franz vertical diffusion cell recommended by USP<1724> |
| Drugs of choice | A testosterone gel preparation(the blood-concentration time curve of this drug is known.) |
| Synthetic membrane | Two different materials |
| The receiving medium | A PH 7.4 phosphate buffer solution containing three different concentrations (0.01%SLS, 0.05%SLS, 0.1%SLS) of sodium dodecyl sulfate |
| Temperature | 32℃±0.5℃ |
| The sampling time points | 0、2、4、8、12、16、24 hours |
| Sample size | 1.5mL |
Experimental results
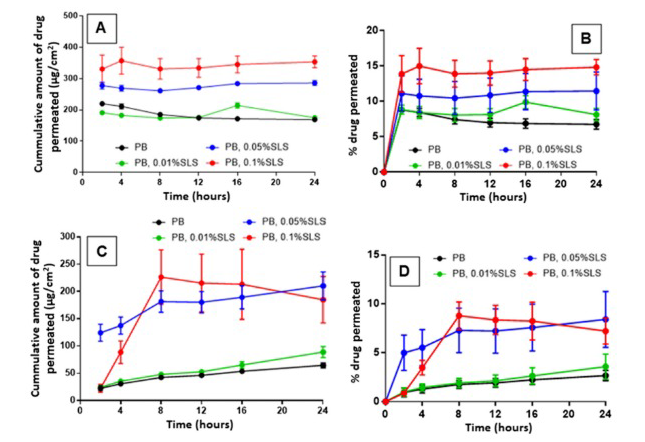
Test Results The IVPT test results were compared with the known blood drug concentration-time curve, and the Cmax and AUC0-24 were used to determine whether the in vitro permeability of the synthetic membrane was similar to that of the known in vivo data. The results show that different concentrations of receiving media all affect the total cumulative permeation volume of the drug. Compared with Figure C, synthetic membranes of different materials have different effects on the permeation volume of the drug under the same concentration medium, with the maximum difference being 2.6 times
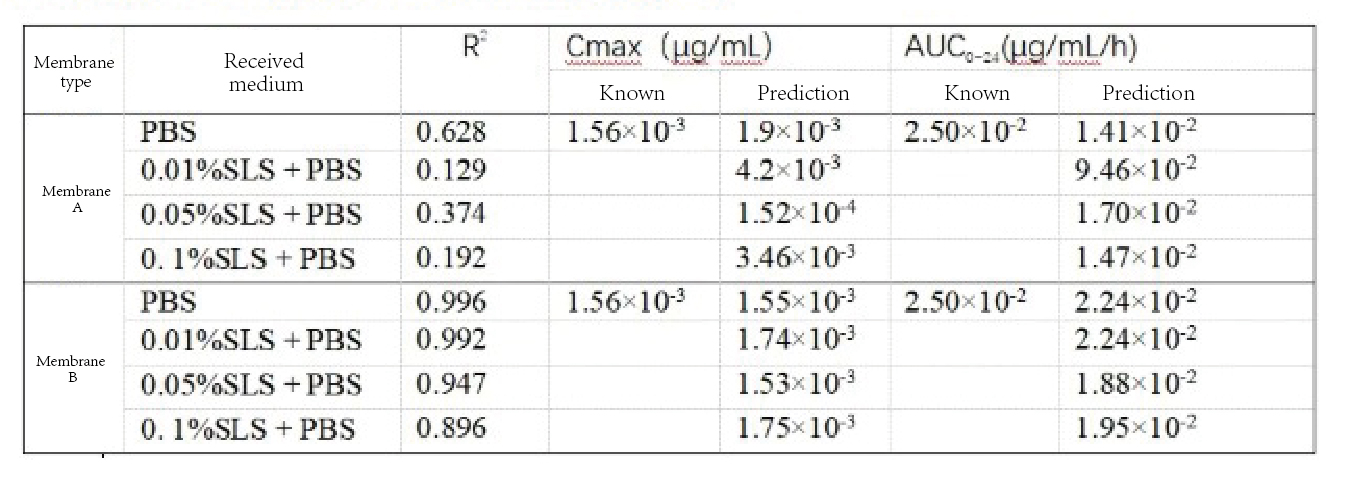
In addition to the analysis of drug permeability, the analysis of Cmax, AUC0 to 24 can also better demonstrate the in vivo correlation. The results are shown in the following table. Different membranes have different biological correlations in the in vivo model, and membrane B has a better correlation. With the increase of the concentration of the receiving medium, the Cmax and AUC0-24 correlations will decrease, which indicates that in terms of the choice of the receiving medium, different media will also affect the quality of the in vivo correlation.
Conclusion:
Therefore, for the biological relevance of drugs, it is worth considering that appropriate synthetic membranes and received media can be used to construct IVPT, so as to have a preliminary prediction of drug performance in vivo, which can reduce the risk of clinical trials in the later stage. For IVPT, good stability and repeatability are the fundamental for biological prediction. Only good instruments can contribute to the development of drugs.

