RT800 Automated Transdermal Diffusion System: Building a core barrier assessment tool for quality control of transdermal drug delivery systems
2025-08-04

In the quality control of transdermal drug delivery systems in the pharmaceutical industry, the transdermal diffusion meter plays a key role as a "drug permeation monitor" - it is not only the necessary testing equipment for the research and development of transdermal drug delivery systems, but also the key equipment for the quality control of transdermal drug delivery products, which has a direct impact on the delivery efficiency and skin safety of drugs. Transdermal diffusion instrument is like a "simulator of skin barrier function", through the determination of the penetration rate of active ingredients in the in vitro skin model, which directly affects the effectiveness assessment of semi-solid preparations, generic transdermal absorption consistency evaluation and other key aspects.
Why testing of semi-solid formulations should not be compromised?
1) Statutory Hardware
The Chinese Pharmacopoeia 2025 edition of the dissolution and release assay adds diffusion cell method as the test standard for semi-solid formulations.FDA requires that the 90% confidence interval for the ratio of the drug release rate of the subject formulation and the original developer in IVRT should be between 75% and 133%, and that the confidence interval for the calculated results of the data of the subject formulation and the original developer in IVPT should be between 80% and 125%. The rest of the national pharmacopoeias list in vitro release and in vitro permeation testing as generic quality hurdles. From the relative standard deviation of IVRT to the confidence interval of IVPT for extended-release drugs, each step is a passport for drug registration and marketing.
2) Patient Lifeline
The rate of release and the amount of penetration of a drug are a balancing act between efficacy and risk: too fast a rate and the safety threshold may be breached; too slow a release and the window of efficacy will not be reached. Too little penetration and the drug may not be effective; too much penetration and it is counterproductive. Each time the data is monitored, the patient is being cleared of the potential risk of loss of control in the body.
3) Quality to be controlled
Sample quality control throughout the entire process of drug management:
- R&D end: core reference for method development
2、Production:Key indicators for batch release
- Regulatory end: the bottom line of release for compliance audits.
A single quality oversight can trigger a chain storm in drug management.
Technology Moat of Raytor Transdermal Diffusers

1) Precision volume control: the science between milliliters
Integrated diffusion cell design: increases cell durability;
High borosilicate glass material: Increase the type of durable medium;
High precision volume error: error ≤ 0.3ml.

2) Innovative structural design: a model of uniqueness
The number of "6+1" diffusion pools: reserved pool space, flexible design of experiments.
Modular structure design: shorten the length of pipeline, reduce the probability of pipeline residue
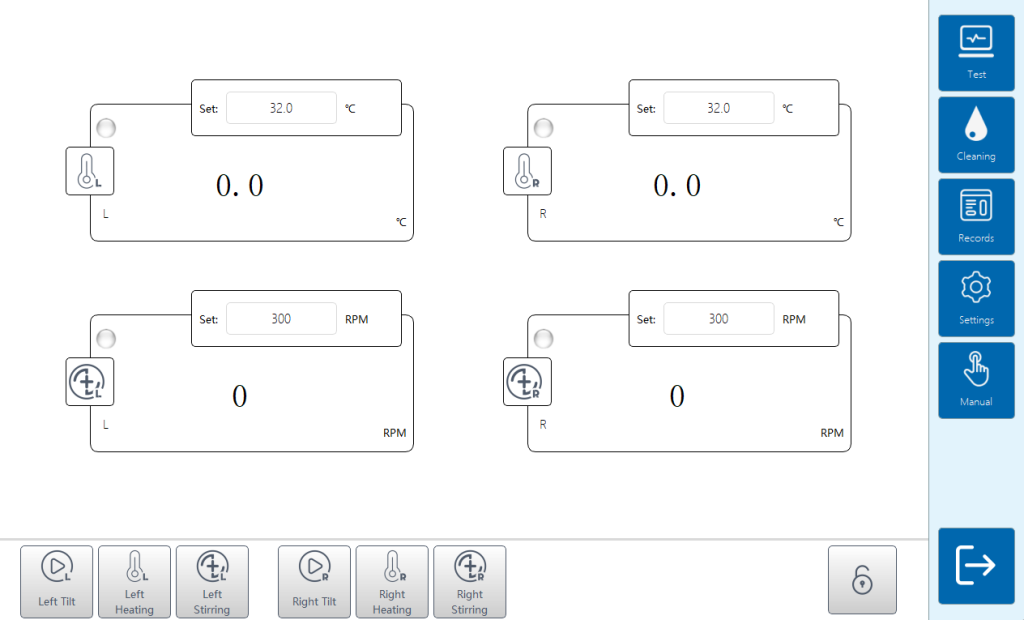
3) Data reliability: building quality from the ground up
Three levels of authority management;
Complete audit trail;
Complete electronic export of lab reports.
Concluding remarks
With the 2025 edition of the Chinese Pharmacopoeia, higher standards are required for transdermal diffusion testing. Automated and intelligent transdermal diffusion systems will become the standard equipment for pharmaceutical companies. Raytor is reshaping the industry benchmark with innovative technology - it is not only the "skin simulation expert" for accurate measurement, but also the "key to pass" to help pharmaceutical companies pass the consistency evaluation. In the wave of upgrading the quality of transdermal drug delivery, what kind of transdermal testing partner to choose may determine the leading edge of enterprises in the innovation track.



