Tablet Dissolution Analysis Services are essential when poor bioavailability stalls development and pushes quality risks into later, costlier stages. At Raytor, we see this: variable release profiles, weak method transfers, and delays triggered by rework. As a manufacturer of pharmaceutical analytical instruments, we built our services and systems to make dissolution results faster, clearer, and easier to trust.

Why Poor Bioavailability Happens
- and How Tablet Dissolution Analysis Services Help
Low or inconsistent bioavailability often starts with fundamentals: formulation choices, medium control, and method execution. Small variations in temperature, speed, or sampling timing compound into big differences in release curves. Compliance pressure adds another layer. Teams must document every step and show data integrity, while still moving projects forward.
Our Tablet Dissolution Analysis Services focus on practical fixes. We begin with pre-formulation or QC context, then design or refine basket/paddle methods that align with national pharmacopoeias. We standardize variables, tighten sampling, and map sources of drift. The outcome is not only a cleaner profile but also a repeatable workflow your team can run with confidence.
Common Pain Points We Solve
• Unstable temperature control and speed drift that distort release curves
• Time gaps at dosing and sampling that increase variability
• Method changes that force hardware reconfiguration and cause downtime
• Pipeline corrosion or adsorption that skews results over time
• Gaps in data traceability that slow regulatory reviews
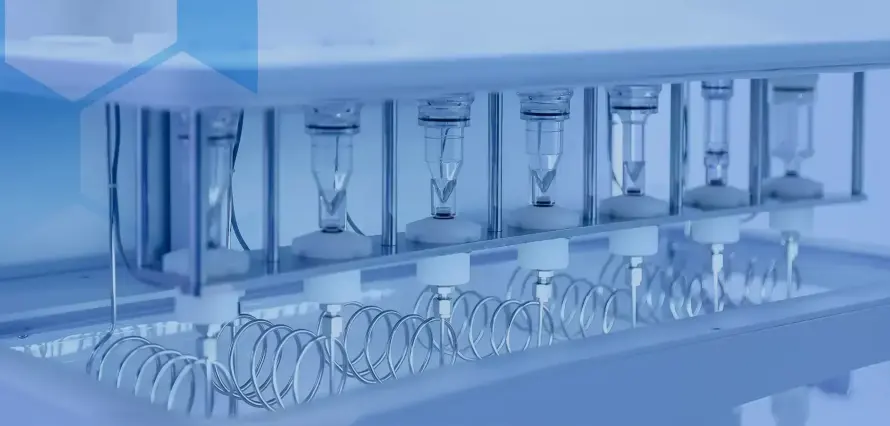
RT612-ST: 12-Position Automated Dissolution Tester
Raytor's RT612-ST is our core platform for service delivery and customer labs. It is a precise pharmaceutical dissolution testing system with 12 positions, designed for both R&D and QC across oral, transdermal, and subcutaneous dosage forms - as well as intrinsic dissolution rate studies of APIs. In practice, this instrument lets us apply Tablet Dissolution Analysis Services with discipline and speed.
The co-axial basket-paddle design supports method changes without re-adjusting unit height. Covered dissolving cups auto-center for quick installation and durability. Three automatic features - medium preheating, filtering, and synchronous dosing - cut manual load and reduce timing errors. A high-precision sampling pump supports dependable, consistent collection, and the Teflon pipeline resists corrosion and adsorption. Dual online filters maintain clarity, while an automatic sample collection module provides a 120-position tray for 1.5 mL vials and a 10 mL test tube rack.
✅ Precision you can measure and audit
• Speed setting range: 20-250 rpm, with 0.01 rpm resolution and ≤ ±0.3 rpm steady-state error
• Temperature accuracy: ≤ ±0.2 °C, with 0.01 °C resolution
• Geometry controls: vessel and shaft verticality 90° ± 0.5°, centering deviation < ±2.0 mm
• Depth positioning deviation: < ±1.0 mm; shaft/basket wobble: < ±1.0 mm
• Automatic sampling workstation: up to 20 settable samples, 1-20 mL range, first point at 3 min, routine points at 5 min, max working time 720 hours, sampling precision ≤ ±1%
• Operating system meeting FDA 21 CFR Part 11 requirements
These controls matter because they remove guesswork. Accurate rpm, tight temperature control, and synchronized dosing keep runs aligned from batch to batch. Adjustable solvent volume and stirring speed allow method tuning without hardware changes.

How Raytor Delivers
Raytor Instruments has focused on advanced analytical solutions since 2015, covering new compound identification, drug permeability, drug dissolution/solubility, and transdermal diffusion. Our R&D platform brings certified senior pharmaceutical experts together with universities and pharma companies in joint labs. This keeps our Tablet Dissolution Analysis Services rooted in current practice and real production needs.
In service engagements, we work end-to-end: assess the dosage form and regulatory context, select basket/paddle methods, and configure parameters for reliable release testing. We use nitrogen-stable pipelines, flexible sampling parameterization, and real-time control of speed and temperature. The Raytor cloud system can connect at least 250 instruments for intelligent management, enabling centralized oversight and smooth data handling across sites. Combined with a nationwide sales team, global partnerships, and 6S production management, we support fast response and efficient order fulfillment.
✅ Where our services fit best
• Oral tablets and patches in late-stage method confirmation and QC
• Semi-solid preparations and injections where filtration and sampling are critical
• Intrinsic dissolution rate of APIs during pre-formulation optimization
• Method transfers that need traceable, Part 11-ready workflows.
Not every challenge needs a new formulation. Often, it needs a stable method, synchronized dosing, and reliable sampling. By aligning your protocol with the RT612-ST's capabilities - automatic medium preheating at scheduled times, dual online filtering, precise sampling, and optional dual drive - you can shrink variability and raise confidence in every curve.
Call to Action: If poor bioavailability is slowing your program, talk to Raytor. Request a service consult or schedule a demo of the RT612-ST. We will help you design, validate, and scale Tablet Dissolution Analysis Services that get you to consistent release - and to market - faster.



