Prediction of Food effect (FE) of Drugs in the Human Body

Food effects are widely present in oral preparations. As dietary conditions can vary with the quantity and type of food and are difficult to strictly control over the long term, relevant departments encourage the development of drug preparations that are not affected by food. When it is impossible to develop such preparations, good and standardized FE studies can be conducted to explore whether the drug can be taken with food and when/how to take it with food.
Get Free Quote
Experimental parameters
| Experimental conditions | Fasting Conditions | Fed conditions |
| Physiological model/spectral conditions | pH shift model /Accurate algorithm | pH shift model /Accurate algorithm |
| Gastric Physiological conditions | Fasted Simulated Gastric Fluid(pH 3.0) | Fasted Simulated Gastric Fluid(pH 6.0) |
| Half-gastric emptying time | 20min | 20min |
| Intestinal physiological conditions | Fasted State Simulated Intestinal Fluid (pH 6.5) | Fasted State Simulated Intestinal Fluid (pH 6.5) |
| Preintestinal absorption time | 120min | 120min |
| API | API | API |
| excipient | not | not |
Experimental result
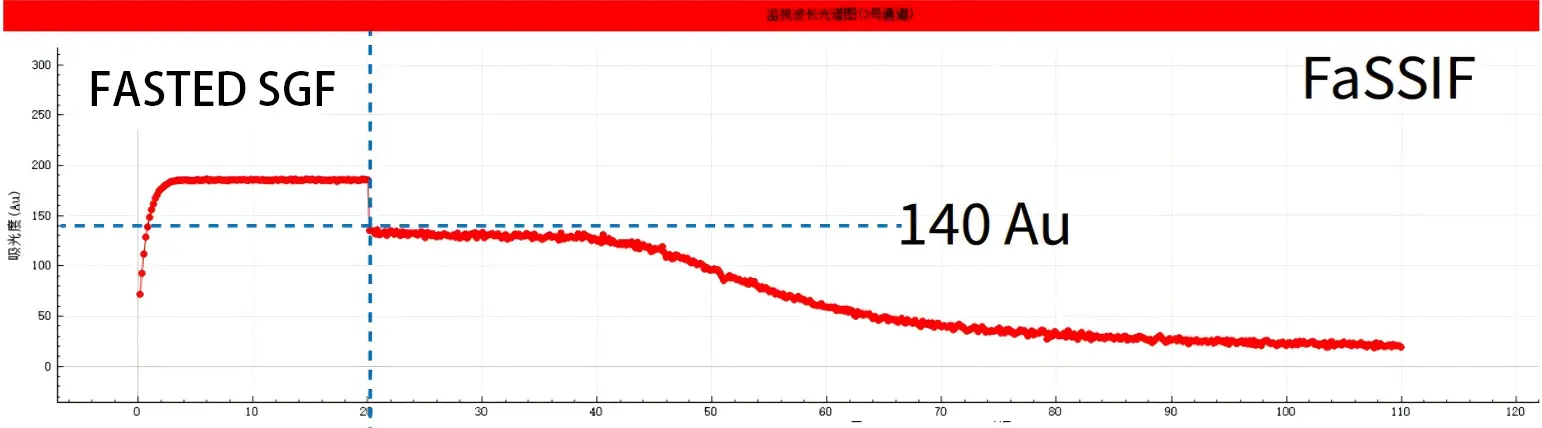
Experimental results In this experiment, we respectively studied the dissolution and absorption of the drug under fasting and fed conditions. The results are as follows: 1) Under fasting conditions, the drug dissolves rapidly (with a peak value approaching 200 AU) within the first 20 minutes of semi-gastric emptying. When switching to the small intestinal segment before fasting (pH6.5), it shows a good supersaturation effect, enabling it to maintain a high concentration in the small intestinal segment without precipitation, and having a large AUC (small intestinal segment area). And the peak value can reach 140Au
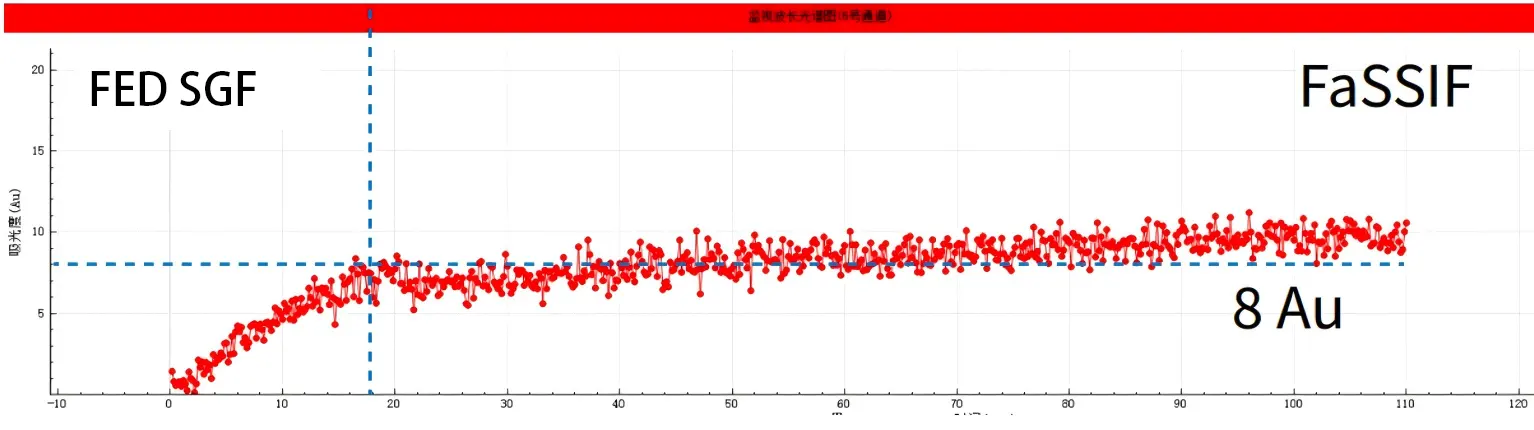
2) Under postprandial conditions, compared with before meals, the drug has difficulty dissolving within the first 20 minutes of gastric emptying (peak value < 10Au). And when switching to the fasting pre-small intestinal segment (pH6.5), the AUC (small intestinal segment area) was relatively small, with a peak value of only 8Au.
Experimental conclusion In the case, NCE DP demonstrated the application of the pH Shift model to study the food effect (FE) required by CDE. The results showed that this APl had a severe food effect (FE), with the peak concentration when taken on an empty stomach being 10 to 20 times that after meals (140 Au:8 Au). Therefore, the clinical application of this drug may cause serious side effects.
Summary
The food effect is a dangerous physiological phenomenon that may cause serious side effects in patients after taking medication (almost all original drugs in Europe and America have undergone food effect studies). Therefore, whether in the development of innovative drugs or generic drugs, formulation experts should have a thorough understanding and study of the impact of this effect, rationally control dosage prescriptions and process parameters, and while further enhancing the authenticity and accuracy of the in vitro dissolution test results of drugs, avoid adverse clinical food effects of drugs, and ensure the safety, rationality and effectiveness of patients' medication. Moreover, when conducting research on food effects, it is important to attach great importance to the standardization and normalization of operations, and to minimize the influence of related excipients and sedimentation phenomena on the test results as much as possible to ensure the acquisition of genuine and effective experimental data.

