Apparent dissolution of APIs in compound preparations
Compound preparations are single formulations composed of multiple ingredients or components, each maintaining specific proportional relationships. During development, compound preparations encounter formulation technology barriers such as drug component selection, stability, and release rate. Differences in dissolution and release behaviour among components can significantly impact therapeutic efficacy. During compound formulation development, determining the apparent dissolution rates of different active pharmaceutical ingredients (APIs) and investigating how factors such as crystal form, grinding processes, and mixing ratios affect the dissolution and release of each API can provide substantial assistance for formulation process design and optimisation.
Get Free Quote
Experimental parameters
| Dissolution Apparatus | RT700 |
| Cell | Large Cell (22.6mm) |
Experimental result
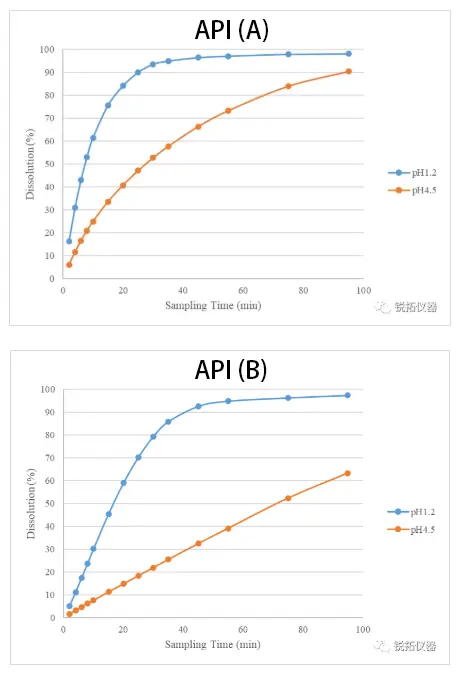
①Selection of Medium: The apparent dissolution rate of a single active API was determined in dissolution media at different pH values to investigate the extent to which variations in release environment pH affect the apparent dissolution rate of the API. The apparent dissolution rate measurements for the two components (API A and API B) in a compound formulation, conducted separately in pH 1.2 and pH 4.5 dissolution media, are presented in the figure below. Test results indicate that the apparent dissolution rates of both active pharmaceutical ingredients exhibit significant pH dependency, accelerating as pH decreases. Furthermore, we observe that the release environment pH exerts a greater influence on the apparent dissolution rate of Active Pharmaceutical Ingredient B.
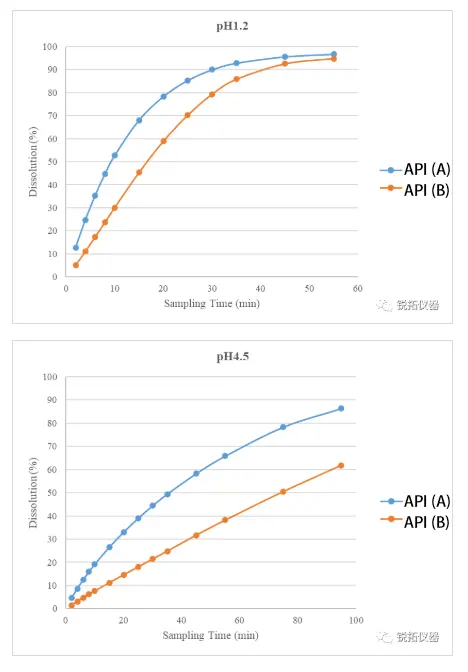
②Apparent dissolution rate of two APIs in a mixed state The two active pharmaceutical ingredients were blended according to the dosage ratio specified in the compound formulation prescription. The apparent dissolution rates of both ingredients in different pH dissolution media were then measured in their blended state. The test results are presented in the figure below. Under varying pH conditions, the apparent dissolution rate of API A consistently exceeded that of API B. As the pH increased from 1.2 to 4.5, the disparity in apparent dissolution rates between the two APIs became more pronounced. Furthermore, no significant difference was observed between the apparent dissolution rates of the two APIs in the mixed state and in their individual component states. This preliminary finding suggests that the two components do not significantly influence each other's dissolution and release rates.
CONCLUSION:
Traditional dissolution methods (basket method, paddle method) exhibit significant limitations when determining the apparent dissolution rate of active pharmaceutical ingredient (API) powders. For instance: API powders may float on the liquid surface after dispensing (paddle method); API powders may leak through the mesh openings of the rotating basket (basket method); soluble APIs may release rapidly within the dissolution vessel, preventing the generation of a dissolution curve; insoluble APIs may accumulate at the vessel base, slowing the overall dissolution rate and diminishing the assay's discriminatory power. Thanks to recent innovations and advancements in flow-through dissolution apparatus, we can now employ more scientifically rigorous analytical methods to study the apparent dissolution of multiple active pharmaceutical ingredients within compound formulations. Furthermore, leveraging the proprietary rapid sampling technology of the RuiTuo RT7 flow-through dissolution system, sampling intervals can be achieved as frequently as every 30 seconds. This enables more precise determination of the apparent dissolution of rapidly released, highly soluble active pharmaceutical ingredients.

