-

ARAB LAB LIVE Dubai | Raytor Instruments RT600 Shines in Dubai and the Exhibition Concludes Successfully!
-
Precise Insight, Risk Avoidance: RT700 Flow-Through Cell Dissolution System Helps You Lock the Consistency Evaluation Victory in Advance-Diclofenac Sodium Extended-Release Tablets Application Case
-

Construction of Raytor's Jiaxing campus is progressing well, moving towards a new chapter in the future!
Precise Insight, Risk Avoidance: RT700 Flow-Through Cell Dissolution System Helps You Lock the Consistency Evaluation Victory in Advance-Diclofenac Sodium Extended-Release Tablets Application Case
2025-09-26
Preface
In the long journey of generic drug consistency evaluation, have you ever encountered such a dilemma: you have invested a huge amount of money to complete the BE test but accidentally failed, and then realized that the root cause is the lack of differentiation power of in vitro dissolution method, and failed to detect the intrinsic differences between the product and the original research? This is especially critical for diclofenac sodium extended-release tablets, which are extremely sensitive to release behavior. The ideal in vitro release profile should accurately mimic the gradual release of the drug in the human gastrointestinal tract as it undergoes a pH change (from an acidic gastric to a neutral intestinal environment), and any mid- to late-stage release deviation may indicate a significant risk of BE failure.
② Case background and results
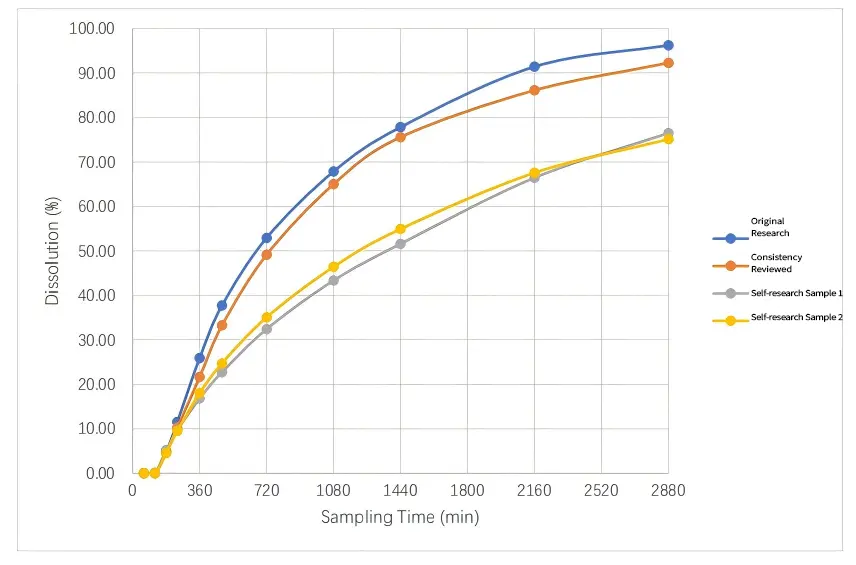
In this test, the test applicant wished to evaluate the release behavior of two batches of its homemade samples under the conditions of the Flow-through cell method, which is closer to the physiological environment of the human gastrointestinal tract. The experiment was conducted in open-loop mode - supporting multiple pH auto-switching (running at pH 1.2 first to simulate the gastric environment, and then automatically switching to pH 7.2 to simulate the intestinal environment), aiming at comparing the consistency of the release behavior of its product with that of the originator and another evaluated product under the mature methodology and close physiological conditions, so as to provide a key basis for decision-making on the BE test. The test results are as follows:
✔ The dissolution profiles of the over-evaluated product and the original drug were highly similar (f2 = 76.78 > 50), which verified the reliability of the method.
✔ The two batches of self-researched samples, although with good batch-to-batch consistency, both showed significant differences from the original and the over-rated product: the dissolution rate was significantly slower than that of the original drug 240 min after the start of the experiment, and the final dissolution was only about 76%, which was much lower than that of the original drug (96.23%) and the over-rated product (92.26%).
✔ This result serves as an accurate "in vitro BE preview". It is a clear warning that the current prescription process (e.g., selection or ratio of sustained-release materials) may result in incomplete release of the drug in the intestinal environment and insufficient bioavailability. The probability of failure is extremely high if the BE test is performed directly.
③Raytor RT700 Flow-Through Cell Dissolution System Advantages

Raytor RT700 Flow-Through Cell Dissolution System
This study relies on the core technology of RT700 Flow-Through Cell Dissolution System, which perfectly fits the testing challenges of extended-release formulations:
✔ Pioneering Flow-through cell method device with one-button switching between open and closed loops: fully automated media switching, precise simulation of pH changes in vivo, seamlessly reproducing the in vivo release journey of diclofenac sodium extended-release tablets.
✔ High-precision syringe pump: provide 0Hz pulsation-free flow, especially suitable for slow-release formulations, avoiding fluid shear interference with gel layer or drug release structure, obtaining more realistic release data.
✔ Fully immersed water bath: Ensure the flow cell and 2.5L large-capacity solvent bottle are in a precise constant temperature environment of ±0.2℃ throughout the entire process, providing sufficient solvent and absolutely stable temperature guarantee for the release preparation for more than 12 hours.
Conclusion
Consistency evaluation, success or failure lies in the details, and the key lies in foresight. RT700 Flow-Through Cell Dissolution System, with its highly physiological environment simulation, intelligent and flexible automation design, and stable and accurate temperature control technology, provides you with more scientific and more differentiated in vitro evaluation solutions. Utilizing RT7 for method development and risk assessment at the early stage of a project will significantly reduce the risk of unpredictable failures and give you more confidence in your R&D decisions!
