Transdermal Permeation Models should make patch and cream testing predictable. Yet many studies go wrong for simple reasons. Data drifts. Replicates disagree. Timelines slip. A temperature that shifts by a fraction. A stirrer that runs unevenly. A fill that traps a single bubble. Each looks harmless. Together they bend the curve and hide the truth. Why do compliant methods still fail? Which five errors cause most rework? And what quick checks prevent them? In the next section, we open the lab notebook and show where performance really leaks.

Where Transdermal Permeation Models Go Off-Track - and Why It Matters
Most problems start before the sample ever meets the membrane. A water bath runs a fraction hot. Stir speed differs between cells. Media height is set "by feel." A tiny bubble sticks to the membrane and blocks the diffusion path. Each issue looks harmless in isolation; together, they bend a promising profile into noise. Teams repeat runs, extend timelines, and spend budget chasing stability instead of learning from the formulation.
Small operational slips create big scientific consequences. When instruments are bulky or fiddly, operators adapt. Steps become "close enough," notes get thin, and methods turn interpretive. That is when Transdermal Permeation Models stop reflecting formulation behavior and start reflecting apparatus artifacts. The fix is not magic. It is disciplined control of temperature, rotation, media level, and interface quality - executed the same way, every time.
- Regulatory and commercial impact
Sponsors and partners judge results on traceability and comparability. Studies that follow USP <1724> conventions for in vitro release and permeation get traction faster. Confidence grows when conditions are stable and well documented, so review cycles shrink and scale-up decisions come sooner. Strong, standard-aligned Transdermal Permeation Models reduce re-work and protect program schedules.
- Process discipline beats complexity
We've learned that simpler tools make better data. If the apparatus is straightforward, training is short, hand-offs are clean, and operators stick to the script. Consistency across shifts and sites matters more to decision quality than exotic features that rarely get used. A clear, repeatable workflow - especially during Franz diffusion cell testing - is often the single biggest driver of reproducibility.
Five Fixable Errors RAYTOR Sees Every Week
Even experienced labs stumble on the same routine details. Here are the mistakes we're asked to troubleshoot most often - and practical ways to prevent them inside Transdermal Permeation Models.
• Drifting temperature or rotation. Tiny swings in either variable change flux more than most teams expect. Set both centrally, verify across all cells, and log during the run. Stability beats precision you cannot hold.
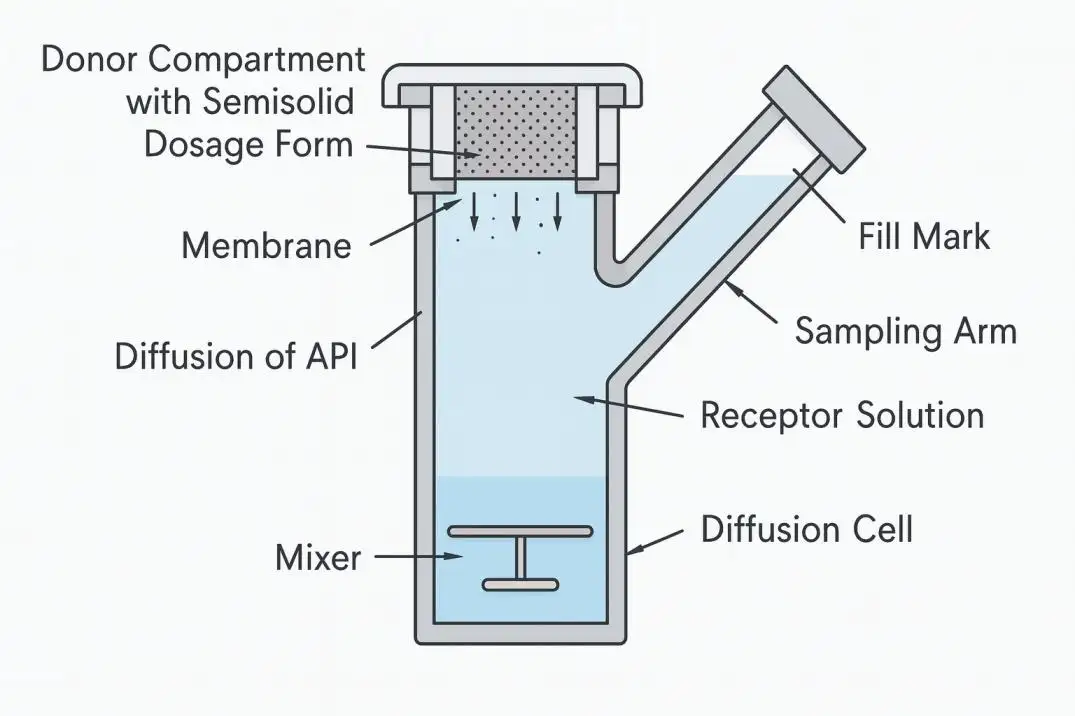
• Bubbles at the membrane. One bubble blocks transport and underestimates permeation. Use a filling-point workflow: first clear bubbles, then inject medium only to the marked level so bubbles do not reform.
• Deviations from USP <1724> essentials. Cell type, media handling, and rotation control must match the described method. Document settings, not just nominal targets, and avoid improvised hardware combinations.
• Inconsistent media height. Over- or under-filling changes the effective diffusion area and boundary conditions. Mark the fill point and enforce the same sequence on every run.
• Over-complex apparatus and training burden. When setup is hard, steps get skipped under time pressure. Favor an extremely simple structure with manual, visible controls so new staff ramp quickly and veterans stay consistent.
Individually, none of these errors feel dramatic. Collectively, they explain why two identically labeled studies disagree. Remove them and your Transdermal Permeation Models move from "good enough" to decision-grade.
RAYTOR's Practical Fix: Simple Gear, Clean Data
RAYTOR builds equipment to keep science honest without slowing the lab. Our compact vertical Franz diffusion cell approach integrates heating and a built-in stirrer in a portable footprint. It parks easily on crowded benches and supports day-to-day testing without fuss. Temperature and rotation are quick to set and, more importantly, easy to hold steady across cells. That stability underpins reliable transdermal testing.

Our system aligns with USP <1724> for simulating in vitro release and permeation procedures, so your studies rest on a recognized standard rather than a custom workaround. The diffusion cell itself is designed with a clearly marked filling point. You remove bubbles first, then bring the medium up to that mark - this simple, teachable step prevents re-formation of bubbles and yields dependable outcomes over repeat runs. Adjustments for test temperature and stir speed are fast, and the layout stays readable at a glance, which helps operators catch deviations in time to correct them.
We emphasize manual clarity because it improves reproducibility. A straightforward structure reduces cognitive load, cuts training time, and lowers the odds of "close enough" choices that haunt later analysis. When the apparatus is predictable, Transdermal Permeation Models can focus on formulation truths instead of compensating for equipment quirks.
What You Can Test Today
RAYTOR's vertical Franz diffusion cell workflow supports semi-solid preparations within the scope of USP <1724>:
• Creams
• Ointments
• Patches
• Gels
That coverage standardizes methods across teams and sites, making it easier to compare candidates, defend results, and move promising options forward. It also aligns with how most development pipelines actually work: frequent screening early, then tighter confirmatory work as candidates mature. By keeping setup portable and adjustments quick, labs can scale the number of cells in use without multiplying complexity.
For search discoverability, many customers find us while looking for USP 1724 compliant transdermal testing or Franz diffusion cell testing for creams. If those phrases brought you here, you're likely facing the same bottlenecks we described: drifting conditions, inconsistent fills, or recurring bubbles at the membrane. Each is solvable with process discipline and hardware that makes the right action the easy action.
Call to Action
If your Transdermal Permeation Models are drifting - or if repeat runs are burning time and budget - start by simplifying the method. Standardize temperature and rotation. Enforce a no-bubble fill to the marked point. Align your documentation with USP <1724>. RAYTOR can help you implement that workflow fast. Talk to RAYTOR to see how a compact, clear, and compliant vertical Franz diffusion cell setup turns routine testing into repeatable, auditable evidence that speeds decisions and reduces re-work.