-

Intelligent Leadership, Precision and Efficiency: Raytor Instruments Successfully Finishes 2025 National Pharmaceutical R&D Innovation Conference
-
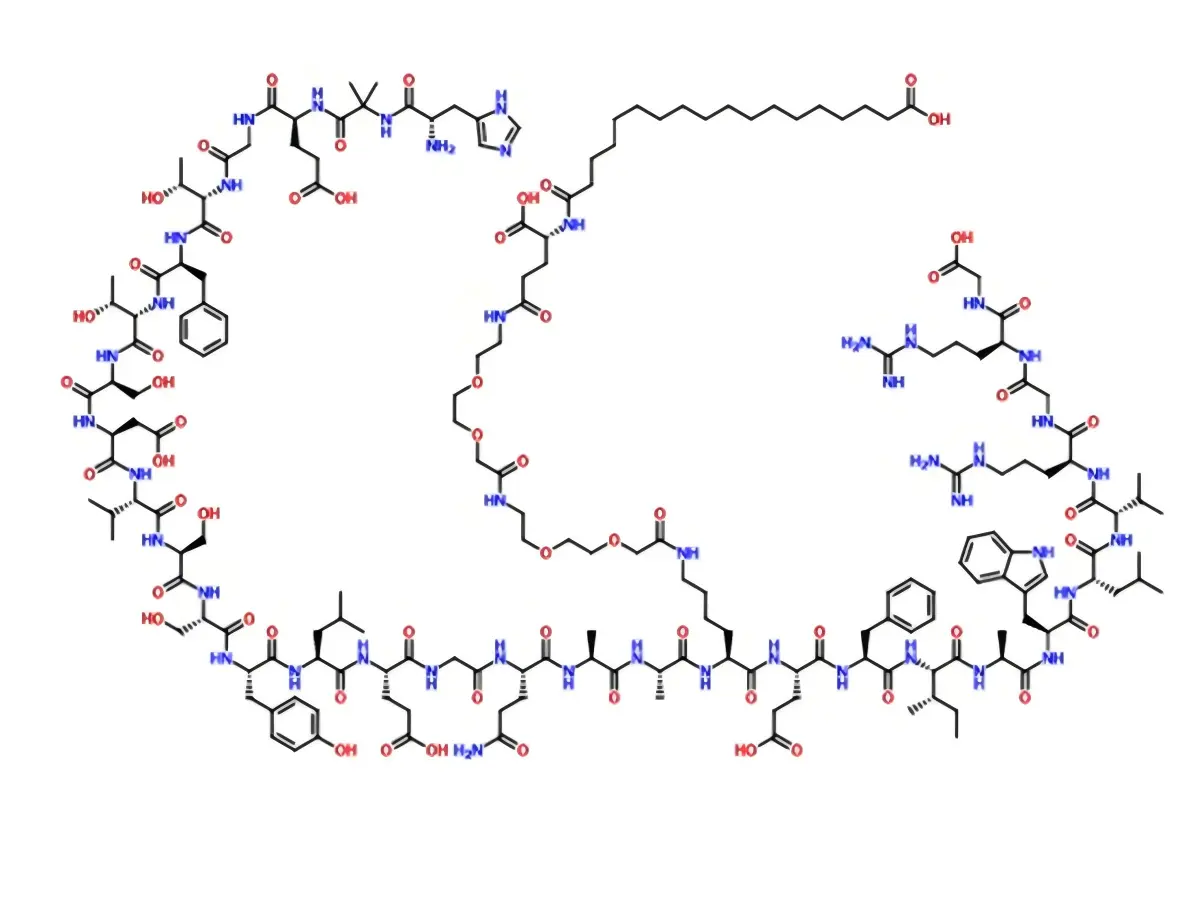
How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
-

What Is Precision Laboratory Automation and Why It Matters
Outdated tools drain time, money, and trust. Raytor Laboratory Equipment offers a clear path to stable, auditable results. Labs replace guesswork with repeatable methods. Downtime drops. Training gets easier. Compliance stops feeling risky. Data tells one story, not many. But the biggest change isn't just new hardware. It's what happens to your workflow, your inspections, and your project timelines. In the next section, we reveal the hidden costs most teams miss, the signals that it's time to upgrade, and the simple steps to move fast - without disrupting a single study. Ready to see what you've overlooked?

Why Outdated Tools Cost More Than You Think
Old instruments slow science. They drift, fail, and produce data you cannot fully trust. Each small inconsistency forces repeats and eats into limited study windows. Spare parts arrive late or not at all. Method validations stretch on because software is clumsy and audit trails are thin. If your analysts still export CSV files to patch together results, you know the risk. One version error or lost file can derail an audit.
Hidden costs stack up. Unplanned maintenance interrupts runs. Manual sampling introduces variability. Cleaning takes too long and still leaves doubt about carryover. When compliance expectations rise - think electronic signatures, role-based access, and complete audit trails - bolt-on fixes cannot keep up.
✅ Common pain points solved by Raytor Laboratory Equipment
- Inconsistent dissolution results between operators or shifts
- Manual steps that invite error and delay decisions
- Weak data integrity and incomplete audit logs
- Lengthy setup, cleaning, and re-validation after changes
- Limited scalability for multi-batch or multi-method work
Reliability is more than a new box on the bench. It is a standardized process that turns methods into repeatable, defendable outcomes.

What You Gain With Raytor Laboratory Equipment
Precision And Compliance, By Design
Dissolution systems from Raytor comply with Chinese, U.S., and EU Pharmacopoeias and cover USP 1 - USP 4 devices. Each system is engineered to meet 21 CFR Part 11 with secure audit trails, controlled user permissions, and electronic signatures. Interfaces are clean and direct. New analysts learn quickly, and seasoned staff set methods the same way, every time. A broad set of dissolution accessories and fast customization reduces method transfer risk and simplifies scale-up. If you are searching for a 21 CFR Part 11 compliant dissolution apparatus, this is it - without the usual configuration hassle.
Transdermal diffusion testing receives the same rigor. Raytor provides manual and automated cell systems that support IVRT and IVPT method development for semi-solid preparations and patches. Automated sampling and optimized cell geometry reduce specimen residue, improving accuracy and repeatability in line with pharmacopeial guidance. For teams screening new compounds, algorithm-assisted software detects drug molecules and helps characterize pharmacokinetics in simulated environments, so you can refine formulations earlier and with more confidence.
Built For Real-World Workflows
Throughput matters. So does data integrity. Raytor Laboratory Equipment supports multi-batch operation, precise media dosing, and automated cleaning cycles that standardize what happens after a run. This removes hand-offs that cause mistakes and frees senior scientists to focus on interpretation rather than routine maintenance.
✅ Workflow advantages you can expect
- Intuitive operation that shortens training and reduces method drift
- Automated sampling and solvent handling to lower variability
- Standardized cleaning sequences that minimize carryover risk
- Integrated data acquisition, processing, and reporting
- Accessories aligned to oral, transdermal, and subcutaneous dosage forms

The impact shows up in the metrics that count: shorter cycle times, fewer deviations, and cleaner reports. Teams move projects forward without re-testing due to instrument drift or manual variability. If you seek an automated transdermal diffusion cell system for consistent IVRT/IVPT, you will find Raytor's approach practical and scalable.
Results You Can Defend
Regulatory pressure is not going away. Sponsors and agencies alike expect traceability and secure data. With Raytor Laboratory Equipment, every step - who did what, when, and how - is logged. Standardized methods reduce operator-to-operator differences. Reviewers gain clarity. You gain the confidence to submit, audit, and move to the next stage without hesitation.
A Partner, Not Just A Vendor
Raytor invests in the full product lifecycle. Our R&D platform, application experiment center, and production operations are designed to turn field feedback into better instruments - fast. A team of 50+ expert researchers and engineers works across 36,000 m² of facilities, collaborating with leading universities and pharmaceutical partners. Joint labs help us test ideas under realistic conditions and build features that matter on day one.
Service is close to the science. A global sales network provides 24-hour rapid response. Engineers understand method specifics and help align accessories, sampling schemes, and data outputs with your protocol. Lean, 6S production management ensures consistent quality and on-time delivery. When customization is needed, we move quickly - so your system arrives ready for validation and routine use, not a long integration project.

Where Raytor Fits In Your Strategy
If you run pre-formulation studies, Raytor Laboratory Equipment tightens method control and increases throughput. For quality control, it brings consistency, clear audit trails, and repeatable setup. In research environments, automation removes routine work and gives scientists more time for analysis. Across dissolution, transdermal diffusion, new compound analysis, and lab automation, the pattern is the same: dependable data, less friction, and faster decisions.
Not every lab upgrades for the same reason. Some teams need capacity now. Others want to reduce deviations before a critical filing. Many seek a foundation for future automation. Raytor meets you where you are, then scales with your pipeline.
Call To Action: If your lab is ready to replace uncertainty with reliability, let's talk. We can map your current methods, identify bottlenecks, and recommend a step-by-step upgrade path - installation, training, and ongoing support included.
Contact Raytor
• Phone: +86 (20) 3160 8901
• Email: sales@raytor.com
• Address: Nanshan District, Shenzhen, Guangdong, China
By moving beyond outdated tools and standardizing on Raytor Laboratory Equipment, your team gains reproducible results, shorter study cycles, and a stronger regulatory posture. Upgrade once - benefit in every batch, and build a lab that keeps up with your science.
