-

Intelligent Leadership, Precision and Efficiency: Raytor Instruments Successfully Finishes 2025 National Pharmaceutical R&D Innovation Conference
-
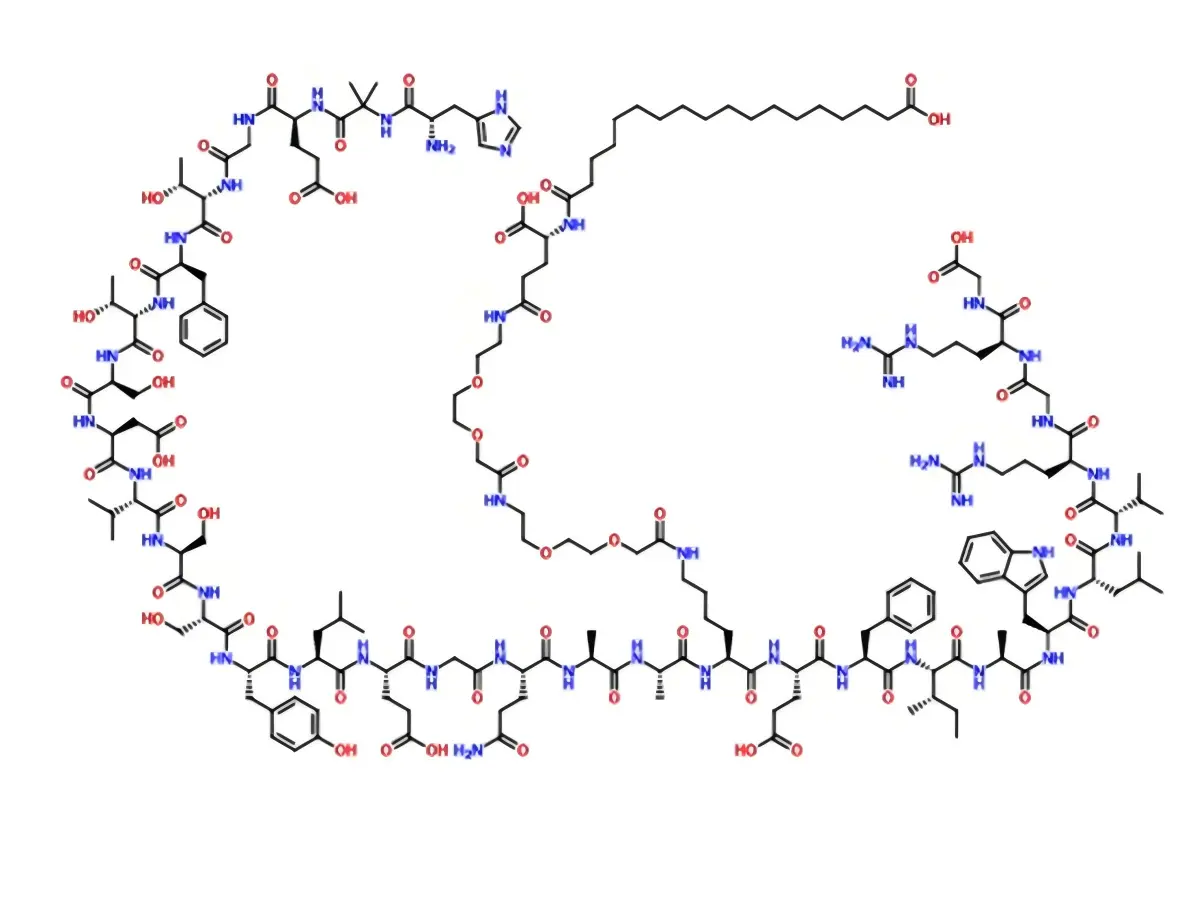
How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
-

What Is Precision Laboratory Automation and Why It Matters
Optimize Skin Drug Delivery Using Reliable Transdermal Diffusion Testing Services
2025-10-15
Transdermal Diffusion Testing Services evaluate how drugs move from topical or patch formulations through skin using IVRT/IVPT methods (e.g., Franz cells). They deliver reproducible permeation data for formulation optimization, quality control, and regulatory submissions. At Raytor - a manufacturer dedicated to the research, development, production, sales, and service of high-quality laboratory scientific instruments - we help R&D and QC teams turn uncertain diffusion signals into evidence they can present to management and regulators. When testing is dependable, development timelines compress, submissions move forward, and routine IVRT/IVPT work no longer clogs the lab.

Reliability Is The Missing Link In Skin Delivery
Skin is not a uniform barrier; it changes with temperature, hydration, excipients, and even handling technique. That is why small imperfections in the test setup can snowball into inconsistent data. Teams wrestling with variable skin absorption, manual sampling drift, or incomplete records often repeat the same runs, spending budget without gaining clarity. Reliability, in this context, goes well beyond accuracy. It means repeatability across days and operators, traceability from method to result, and the throughput needed to explore multiple formulas at once.
Raytor addresses these pain points with automated operations that standardize cleaning, sampling, and refilling. By removing timing guesswork and minimizing manual touchpoints, we reduce operator bias and protect the shape of your release and permeation profiles. Our diffusion cell design follows the USP <1724> compliant style and aligns with the European Pharmacopoeia (EP 9.0) 2.9.4 Dissolution test for transdermal patches, so your data structure mirrors what reviewers expect for semi-solid and transdermal products.
The Cost Of Small Errors
A few seconds of mismatch between parallel cells can distort early time points. Micro-bubbles that go unnoticed will disrupt steady-state flux. Residue in a sampling line can carry analyte into the next draw. None of these issues is dramatic in isolation; together, they erode confidence and trigger rework. With Transdermal Diffusion Testing Services that manage timing, flow paths, and sample handling in a controlled way, you prevent these tiny errors from accumulating.
What Regulators Expect
Reviewers look for coherent methods, consistent execution, and records that connect each result to its conditions. Conformance with USP <1724> and EP 9.0 guidance gives your team a stable framework: appropriate diffusion cell style, defined operating procedures, and documentation that can withstand inspection. The benefit is not just a smoother review - your internal decisions become faster because your evidence is easier to trust.

How Raytor Turns Diffusion Data Into Decisions
Raytor engineers reliability into every part of the workflow - from the way cells are handled to the way data is stored. The result is faster, cleaner, and more defensible diffusion data for Transdermal Diffusion Testing Services.
Cells That Simplify Hands-On Work
Our compliant diffusion cells provide an open operating space for loading and sampling. A clearly marked filling point shows whether the medium has reached the correct level after bubbles are removed, reducing guesswork and preventing bubble re-entry. Each cell can be lifted out and reseated with minimal effort, making visual checks and adjustments quick during setup or troubleshooting. These practical details cut setup time and reduce off-spec runs.
Throughput Without Timing Drift
Two independent groups of diffusion cells can run at the same time, giving you flexibility to compare formulations, excipient levels, or process conditions side by side. A simultaneous sampling architecture ensures that all pools in the same group are sampled at identical time points. With 6+1 diffusion cells per group, you can assign a blank to screen for interference, then interpret the active groups with greater confidence.
- Up to seven cells per group support efficient method screening and confirmation.
- Synchronized automatic sampling keeps timepoints aligned across replicates.
Automation That Protects Results
Manual sequences are vulnerable to fatigue and timing inconsistencies. Raytor automates cleaning, sampling, and refilling, so the same steps occur the same way every run. A shorter pipeline design helps reduce sample residue, while synchronized multi-channel sampling preserves the temporal fidelity of your IVRT/IVPT curves. These controls matter when you compare prototypes, scale up a winning formula, or perform site transfers that must reproduce results.
Data You Can Defend
Strong science needs strong records. Raytor systems include a complete audit trail backed by an SQL database, with capacity to store no fewer than 200 test methods and at least 100 user accounts. Method versioning, user accountability, and centralized storage make it easier to manage access while keeping your study history intact. When investigations arise, you can reconstruct what happened - who ran it, which method was used, and how the sequence proceeded - without hunting through scattered files.
From Bottleneck To Advantage - Where It Matters Most
When Transdermal Diffusion Testing Services remove friction, development accelerates. Raytor's platform meets the test requirements of USP <1724> for in vitro release and permeation of semi-solid preparations and supports everyday dosage forms used by dermal and transdermal teams.
Real-World Impact Across Dosage Forms:
- Creams & ointments: Characterize release rates, compare lots, and monitor batch-to-batch consistency without timing artifacts.
- Patches: Align IVPT with EP 2.9.4 dissolution concepts to support transdermal system assessments.
- Gels: Evaluate permeation behavior in formulations where rheology can influence flux.
In practice, this means fewer repeats, cleaner concentration-time profiles, and tighter confidence intervals. Method transfers move faster because the process is controlled and well-documented. Investigations take hours rather than weeks, and cross-site teams can rely on the same methods and sampling logic. For teams focused on in vitro permeation testing services for semisolids, these gains translate into fewer delays and a clearer view of what to advance.
What Teams Gain?
R&D gains the ability to screen formulations quickly and discard weak candidates early. QC gains stable methods that are easier to validate and maintain. Regulatory affairs gains data packages that are coherent from setup to result. Business leaders gain predictability in both timeline and cost. In short, reliable Transdermal Diffusion Testing Services become a strategic tool rather than a compliance checkbox.
Adhering to USP <1724> and EP 9.0 (2.9.4) is not about chasing paperwork; it is about creating a test environment where meaningful differences appear clearly. Raytor's compliant diffusion cell style, synchronized sampling, and audit-ready data controls support this goal. Your teams focus on formulation science instead of procedural firefighting, while stakeholders see a test program that runs the same way, every time.
Final Words
Ready to optimize skin drug delivery with inspection-ready evidence? Speak with Raytor about Transdermal Diffusion Testing Services built on compliant diffusion cells, synchronized automatic sampling, and robust audit trails. Let's standardize IVRT/IVPT, reduce rework, and move your best creams, gels, ointments, and patches toward market with confidence.
