Transdermal Delivery Systems move drugs through the skin in a steady, controlled way. They power modern patches and guide how we judge release performance. In simple terms, they combine a diffusion cell, sampling, and temperature control to model what happens on the body. Labs use these systems to read the curve behind every patch. Yet accuracy often slips - tiny bubbles, timing errors, and heat drift all leave marks on the data. Why do small details change big decisions? And how can a better setup lock in clean, repeatable results? Let's unpack the answers - and the fixes that matter most.
Why Dissolution Accuracy Slips In Real Labs
In regulated labs, tiny flaws grow into expensive problems. A few bubbles on the membrane change the early slope. A sampling event that lands 20 seconds off target nudges the curve. A half-degree temperature drift shifts solubility and diffusion. Shared tubing turns into the highway for cross-contamination. Stack enough small errors and your release profiles zigzag instead of align.
From our conversations with QC and R&D teams, three pain points repeat. First, repeatability is fragile when methods rely on manual tilting, manual sampling, or improvised fixtures. Second, investigators waste hours chasing noise rather than root causes. Third, audits demand proof - clear, time-stamped records that match the standard operating procedure. Transdermal Delivery Systems should remove human variability, not encode it.
At Raytor, we design around that reality. We automate the steps that most often introduce variance and engineer the hardware so common error sources cannot sneak in. The result: Transdermal Delivery Systems that make accurate patch dissolution less of an exception and more of an everyday habit.
How Raytor Tightens Every Variable
Raytor builds a fully automated, 14-position Franz diffusion cell platform with a modular architecture. A diffusion cell module, an automatic sampling module, and a medium thermostat work together so control, repeatability, and documentation live in the same place. The system meets the intent of EP 9.0 General Rule <2.9.4> for transdermal patches and USP <1724> for semisolid performance tests, which helps teams align methods across development and QC.
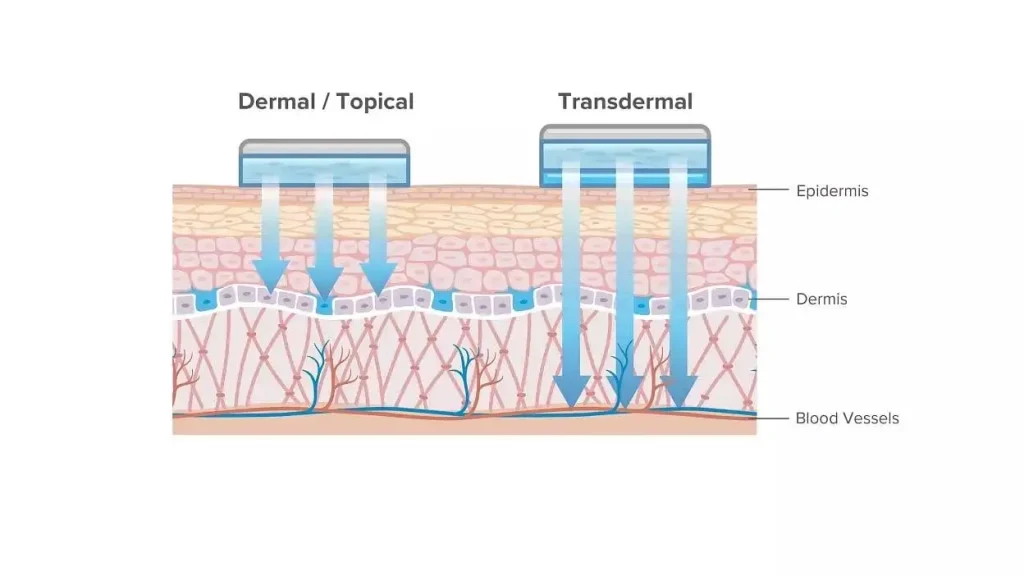
Integrated Cells, Cleaner Hydrodynamics
Long runs punish weak seals and improvised glassware. Our integrated glass diffusion cell structure reduces the chance of weeping leaks that bend cumulative release upward. A unique mechanical motion automatically tilts the cell and drives out microbubbles in real time. That protects the boundary layer and stabilizes early time points, where noise bites hardest.
- Integrated diffusion cell structure minimizes leak risk over long profiles.
- Real-time bubble removal preserves a stable mass-transfer interface.
- Separate pipeline design gives each position dedicated lines and pumps to avoid cross-talk.
Temperature Control Where It Matters
A single bath often hides local differences. Our Transdermal Delivery Systems provide independent temperature control for every diffusion cell. Setpoint control is paired with live monitoring and recording, so you capture actual conditions, not assumptions. When you compare positions or rerun a method months later, you know the thermal story.
- Independent cell-level temperature control reduces between-position variance.
- Real-time monitoring and recording speed investigations with defensible data.
Sampling That Matches Your Method
Sampling should fit the method, not the other way around. Raytor supports partial sampling and full sampling, selectable per protocol. Two independent groups of diffusion cells can run side-by-side without interfering. That means you can test different media, schedules, or formulations in one session, then compare results under identical mechanics.
- Flexible sampling modes (partial or full) to reflect true method design.
- Two independent cell groups enable parallel studies without signal bleed.
- Automated timing removes operator bias from critical draw points.

Designed For Compliance From Day One
Accuracy is inseparable from compliance. By aligning with recognized pharmacopeial chapters for patches and semisolids, our platform gives teams a clearer path through method qualification, routine verification, and audit defense. Instead of stitching together disparate logs, you present synchronized sampling records and condition histories that show exactly what happened and when.
What Accuracy Delivers For Your Team
Better Transdermal Delivery Systems do more than fix a curve; they reshape how work flows across development, tech transfer, and commercial QC. Here's what labs notice first.
More Consistent Curves, Fewer Repeats
When hydrodynamics are stable and temperatures are matched cell-by-cell, release profiles tighten. Early-phase points stop drifting because bubbles cannot linger. Confidence intervals narrow across all 14 positions because each position is truly comparable. You spend less time debating whether a spike is “real” and more time learning from the formulation.
• Fewer out-of-trend profiles and repeat runs
• Narrower inter-position variability during method transfer
• Clearer differentiation between candidate formulations
Faster Investigations, Stronger Records
Investigations are cheaper when the data trail is complete. Real-time temperature and sampling logs let you answer the hard questions quickly: Did position 6 run hotter? Was a sample drawn late at 240 minutes? Are we looking at carryover or a true release feature? With synchronized records, you can separate method faults from material behavior in hours, not weeks.
• Audit-ready logs that match SOP steps
• Traceable, time-stamped sampling and condition data
• Shorter root-cause analysis cycles
Parallel Workloads Without Cross-Talk
Two independent groups of diffusion cells unlock new scheduling options. You can run method development on one side and routine QC on the other. Or compare two membranes, two media, or two agitation protocols in a single day under the same mechanics. Throughput rises, but rigor stays intact.
• Parallel studies for patches, creams, ointments, and gels
• Comparable results under identical hardware conditions
• Less idle time and better use of analysts and instruments
Practical Wins You'll See In Week One
• Cleaner, more repeatable dissolution curves across operators and shifts.
• Reduced manual touchpoints, which lowers handling error and contamination risk.
• Easier tech transfer because mechanics and data capture are standardized.
Why This Matters For Patients And Programs?
Accurate dissolution is not a paperwork exercise. It is the bridge between a controlled design on the bench and steady drug delivery on the skin. When Transdermal Delivery Systems hold the line on mechanics - no leaks, no hidden bubbles, true temperature control - you can trust the curve. That trust accelerates go/no-go decisions, de-risks submissions, and supports consistent patient outcomes once products reach the market.
As a manufacturer, Raytor's goal is to make precision ordinary. We focus on the fixtures you touch every day, from the way a cell seals to how a sample is timestamped. By tightening those details, we help your science speak more clearly - whether you are optimizing a long-wear patch, verifying a semisolid release profile, or preparing a cross-site method transfer.
Final Words
If your team is ready to sharpen patch dissolution accuracy and streamline compliance with EP 9.0 <2.9.4> and USP <1724>, talk with Raytor. Request a demo and a method-fit discussion. We'll review your workflow, pinpoint variability hot spots, and map a path to cleaner curves - and cleaner audits - with Transdermal Delivery Systems built for the way real labs work.



