-

Intelligent Leadership, Precision and Efficiency: Raytor Instruments Successfully Finishes 2025 National Pharmaceutical R&D Innovation Conference
-
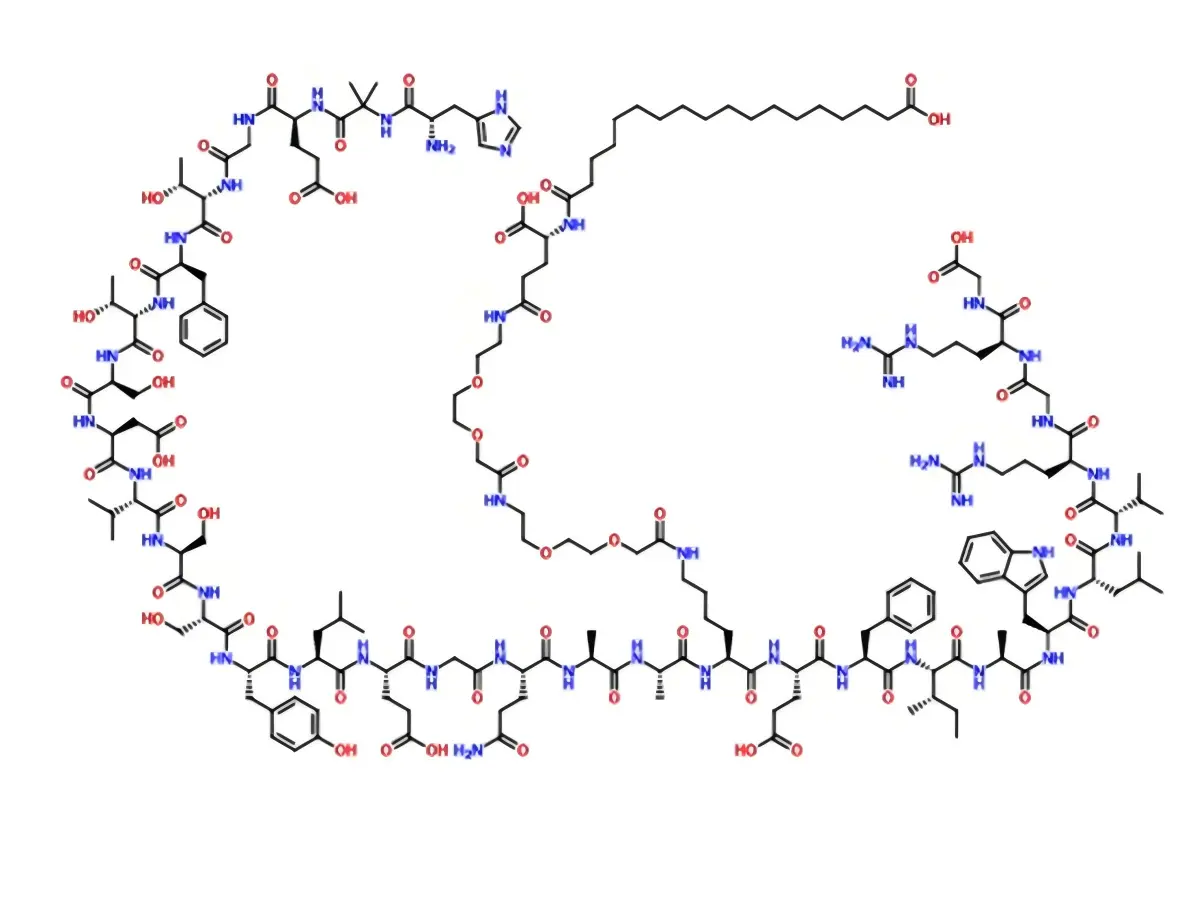
How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
-

What Is Precision Laboratory Automation and Why It Matters
Setting the Stage: The Importance of Dissolution Testing
Dissolution testing is very important for medicine quality. It shows how fast and how well a drug dissolves in the body. This helps us know if the medicine will work the right way. If the drug does not dissolve properly, it may not help the patient. This kind of testing checks if each tablet or capsule gives the same result. It looks for even drug release from one batch to the next. That means every pill you take should act the same. This builds safety and trust.
USP dissolution methods give clear rules for how to do this testing. These rules help companies follow the same steps in every country. That way, the medicine stays safe and effective everywhere. But how does medicine stay the same in every dose? Why USP dissolution methods are a big part of that? Let's explore more together!
The Charm of Consistency in Pharma
Consistency Builds Trust
Patients need medicine they can count on. Doctors and pharmacists do too. When a drug gives the same result every time, people trust it. This is true for both generic and brand-name products. Both need to work the same way in the body. That is why consistency matters. It helps everyone feel safe.
Testing Methods Reduce Risk
Every batch of medicine should work the same. But sometimes there are small changes. These changes can lead to problems. So, companies use the same test methods for every batch. When everyone follows the same steps, it is easier to find mistakes. This lowers the chance of recalls. It also keeps products stable and safe.
Data Helps Approval
Regulators need strong proof before they approve a drug. They look at the test results. If the data is solid, the approval process is faster. Also, the drug gets to the market more quickly. Reliable data also helps when the product goes to other countries. It makes it easier to get approval in more places. USP dissolution methods help create this strong data.
USP Methods: Gold Standard of Assurance
What USP Does
The United States Pharmacopeia, or USP, gives clear steps for drug testing. These steps are based on science. USP makes sure everyone uses the same testing rules. This helps labs follow the same process everywhere. The goal is to make testing fair and correct.
Methods I to VII
USP dissolution methods include several tools. These are called Apparatus I to VII. Each tool works for a different kind of drug. For example, some methods test tablets. Others are for capsules or special coatings. This makes the methods useful in many cases. Companies choose the one that fits their product best.
Why USP Compliance Matters
When a company follows USP dissolution methods, it shows care and quality. It means they want to meet high standards. Many countries accept these methods. So, using them can help a drug company sell worldwide. Also, it builds a good name for the company. People trust them more when they see the USP label.
Raytor: A Global Leader in Dissolution Excellence
Trusted Around the World
Raytor is a company people trust. We are known for smart solutions in pharmaceutical testing. Since 2015, we have worked hard in this field. We focus on many areas. These include new compound testing, drug solubility, drug permeability, and skin diffusion tests. We help labs with tools they can use every day. Our goal is to make testing simple and clear.
Smart Production Methods
Raytor follows strong production rules. We use the 6S method in our factory. This helps us make better products in less time. We meet customer orders quickly. We also improve how we work step by step. Each team checks their work for safety and accuracy. So, every device we make is dependable and ready to use.
Strong Global Sales Team
Raytor has a wide sales network. We have teams in many cities. They give fast support, sometimes in just 24 hours. We also grow our business in other countries. We work with new partners in different regions. This helps us bring our products to more labs worldwide. Wherever testing happens, we are ready to help.

Introducing the RT600 Dissolution Apparatus
Compact and Reliable Tool
The RT600 is a small but powerful device. It helps test how drugs release into the body. It is also used for creams, gels, and patches. This tool follows USP dissolution methods, including USP <711>. So, it is very useful for labs that want to follow trusted steps.
Accurate and Simple Features
This system makes testing easy. It warms up before the test begins. It has covered cups that keep the samples safe. It gives the same amount of drug at the same time. This means each test gives similar results. It saves time and gives clear answers.
Designed for Steady Results
The RT600 is made with smart flow control. It keeps water smooth and steady. It stops bubbles from forming. This keeps the test stable from start to end. So, the data is more reliable. The design helps remove common problems seen in other systems.
Safe Data and Quality Checks
The RT600 can hold a lot of data. It does not limit storage. It also follows FDA rules like 21 CFR Part 11. This means it is ready for use in serious drug testing. It helps labs meet high quality needs. It supports strong record keeping and safe use.
Technological Precision in RT600 Tester
Automatic Preheating
The RT600 has an automatic preheating feature. It helps the machine warm up the water bath before testing begins. The user can set a specific time to start the heating. The system then begins on its own. This saves time and helps keep the test conditions steady. The warm-up ensures that the temperature is right when the test starts. This improves accuracy. It also supports better control during the test process.
Convenient Sampling
Sampling is simple with this device. It uses a non-resident sampling needle. This helps reduce shaking or strong water flow during the test. The needle moves only when needed. This keeps the test conditions calm and stable. The needle also adjusts based on how much liquid is used. So, each sample is taken at the correct level. This reduces errors. It also helps each result match the expected standard. These features make every step smoother and more exact.
Automatic Synchronous Dosing
The RT600 gives doses at the same time for every test. It removes any delay between samples. This is important because small timing changes can affect the results. The system tracks when each dose is given. So, the data from each sample is more trustworthy. This helps create reliable and repeatable results. It also helps follow USP dissolution methods more closely. The device makes sure every dose is fair and on time.
Charming Consistency in Action
Support for Manufacturers
Manufacturers often worry about quality. They want to be sure every batch of medicine meets the same standards. When they use the RT600, they can trust the results. Each test follows a clear process. The system helps lower the risk of mistakes. It also saves time in daily work. Raytor gives strong support with each device. Our team helps users understand and use the system well.
Partnership with Raytor
Raytor works closely with its customers. We help companies keep their testing reliable. We listen to user needs and offer fast help. When problems come up, our support team is ready. The RT600 and Raytor work together to bring smooth, clear testing. USP dissolution methods are at the center of this process. They guide how we build and support our tools.
Consistency as a Standard
The RT600 helps bring the same result in every test. This kind of repeatable data is key in pharmaceutical work. It builds trust. It keeps patients safe. Raytor and the RT600 stand for this kind of quality. We believe that this USP dissolution methods help make consistency possible for every lab, every time.
Ready to Test Smarter with This USP Dissolution Methods?
Use the RT600 to make your work faster and easier. Get steady results every time. Follow USP dissolution methods with full control. Cut down errors. Save time in your lab. Raytor gives strong tools and quick help. Start with a system you can trust. See how simple testing can be. Let the RT600 help you today.
Want to try it in your lab? Let's talk.
