Bipha-sic drug delivery system

Bipha-sic drug delivery systems comprise two distinct release phases, categorised according to their release characteristics as immediate-release/sustained-release, immediate-release/controlled-release, or immediate-release/timed-release formulations. These systems enable regulation of drug release rates, reduction of adverse effects, alleviation of gastrointestinal irritation, and enhancement of bioavailability, thereby providing clinicians with more flexible dosing regimens.
Get Free Quote
Experimental parameters
| Dissolution Apparatus | RT700 |
| Model | Open-loop |
| Cell | Large Cell (22.6mm) |
Experimental result
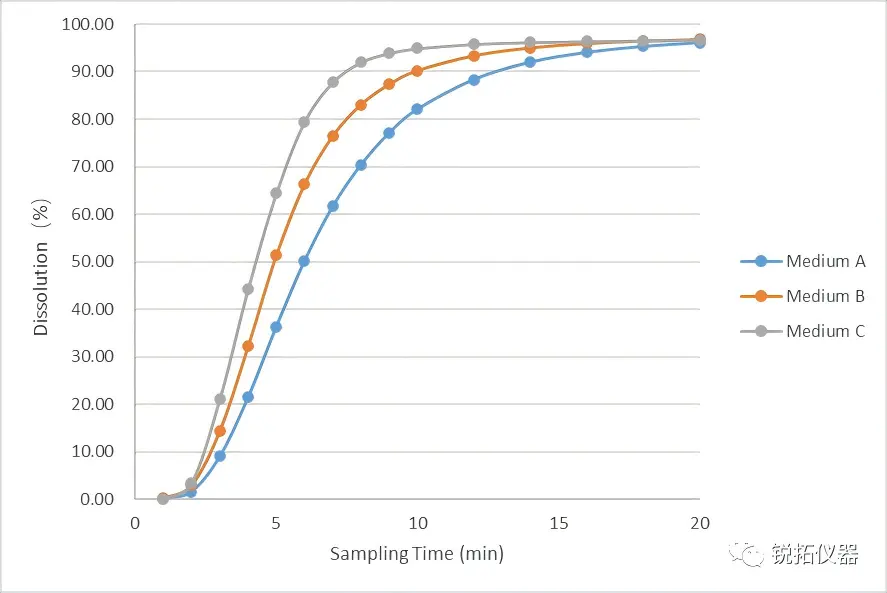
①-01 Selection of Medium and Flow Rate: In this study, we evaluated the effect of varying surfactant concentrations (Medium A < Medium B < Medium C) on the release extent of the formulation within the same pH buffered system. Test results demonstrated that the release rate of the immediate-release components markedly accelerated as the surfactant concentration in the dissolution medium increased.
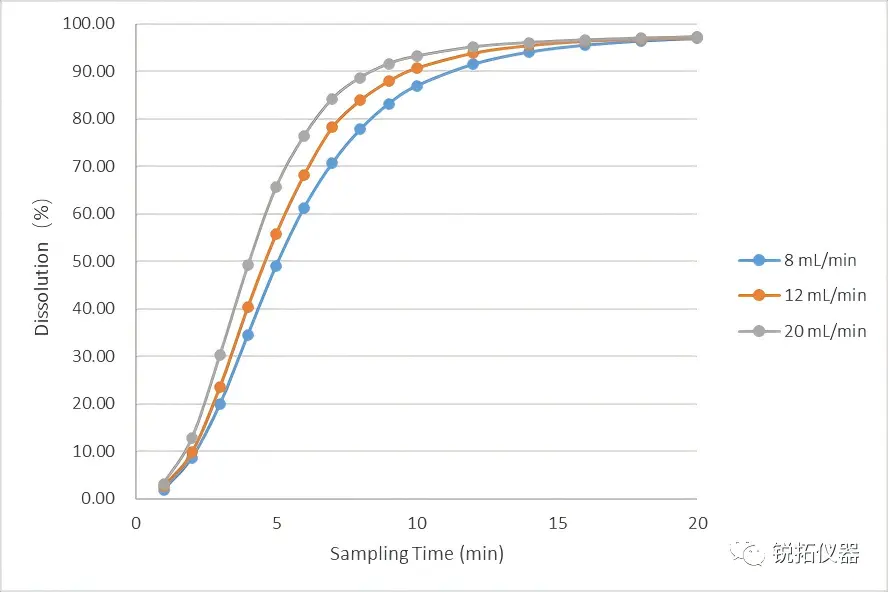
①-02 Subsequently, we evaluated the effect of varying flow rates on sample release under identical dissolution medium conditions. Test results demonstrated that higher flow rates within the dissolution medium accelerated the release rate of the immediate-release components.
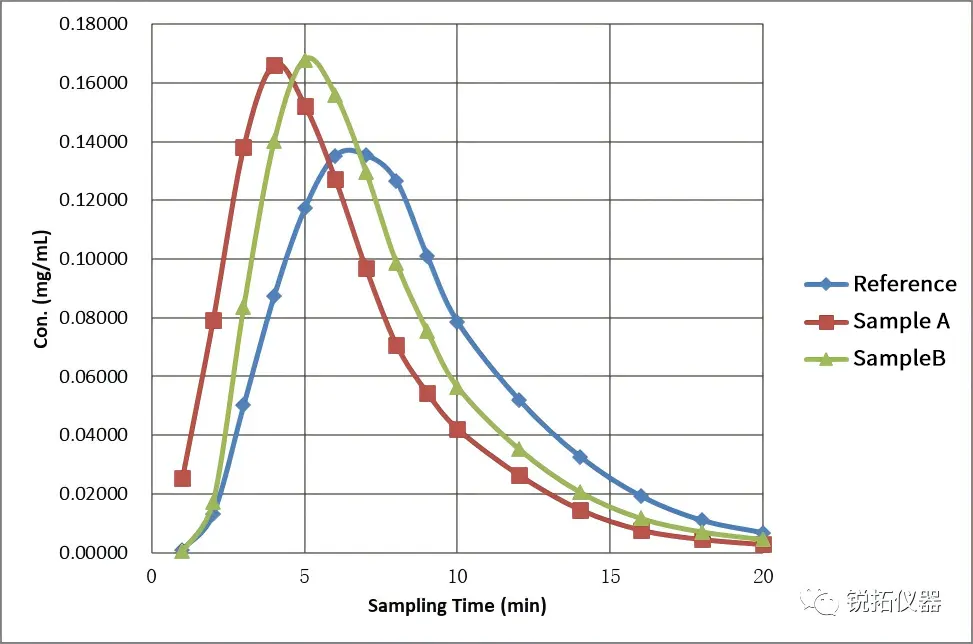
②Distinguish:To determine the discriminatory power of the method, we conducted comparative studies using self-developed samples with different quality attributes alongside the reference preparation. We observed that: (1) For two self-developed samples with distinct quality attributes: Sample A exhibited a faster release rate than Sample B. (2) For the reference preparation and self-developed samples: the reference preparation exhibited a lower Cmax than both self-developed samples, and its time to reach Cmax (Tmax) was slower than that of both self-developed samples.
CONCLUSION:
This dissolution testing method effectively distinguishes differences in in vitro release rates between self-developed samples and reference preparations with varying quality attributes. It provides robust data support for subsequent formulation and process parameter optimisation.

