Increasing the dosage of a drug
The conventional idea is that if the therapeutic effect of a drug in vivo is not good, most people will choose to take more medicine or increase the dosage, and the therapeutic effect of the drug in the body will be improved. However, in fact, in some clinical cases, it has been found that increasing the dosage does not make the therapeutic effect of the drug better; instead, it makes it worse.
Get Free Quote
Experimental parameters
| Experimental design | The pH-shift model in Raytor NCE DP | The pH-shift model in Raytor NCE DP |
| Experimental conditions | A team | B team |
| Physiological model/Spectral conditions | pH shift model/Accurate Algorithm | pH shift model/Accurate Algorithm |
| Gastric physiological conditions | SGF | SGF |
| Half-gastric emptying time | 20 min | 20 min |
| Intestinal physiological conditions | FaSSIF | FaSSIF |
| Preintestinal absorption time | 120 min | 120 min |
| Dosage | 100% | 150% |
Experimental results
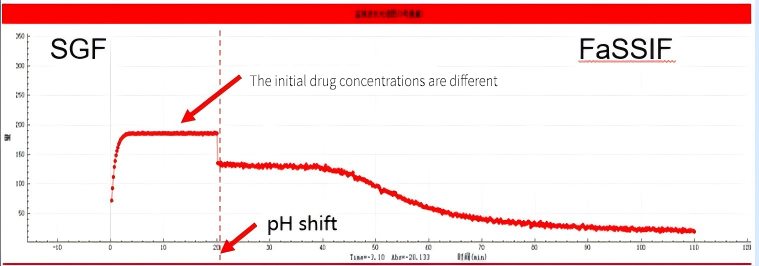
According to the test results, it can be seen that regardless of the dosage, this drug has a good dissolving capacity in the simulated gastric juice. However, when the drug entered the simulated intestinal fluid, different dissolution conditions occurred in the two experimental groups: when group A entered the intestine, although the drug concentration would decrease, it would maintain a steady state for a period of time before slowly decreasing, which is known as the supersaturation effect. This effect can slow down the precipitation of the drug and improve its absorption. However, after Group B entered the intestine, the drug concentration dropped rapidly, and the drug directly existed in the small intestine in the form of precipitate without any supersaturation effect.
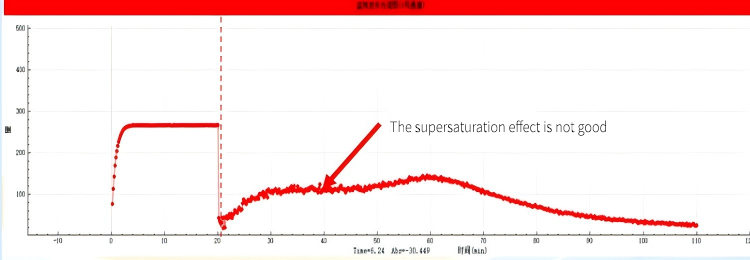
The results showed that the AUC area of small intestinal segment in group A was larger, and the AUC area of this segment was 538 calculated by the self-developed algorithm, while the AUC area of small intestinal segment in group B was smaller, which was 408.
Experimental conclusion
Increasing the dosage of the drug instead causes the drug to exist directly in the small intestine in the form of precipitate, resulting in poor absorption in the small intestinal segment and ultimately manifested as a decrease in the drug's efficacy. This indicates that when the drug's efficacy is poor, simply increasing the administration concentration will not improve the drug's efficacy; instead, it may even have the opposite effect.
Discussion
In the case, NCE DP demonstrated the application of the pH shift model, which is used to study the precipitation effect of BCS2-class alkaline drugs in vivo. Many original research drugs are formulated using SDDS (supersaturated drug delivery systems), and their ability to maintain supersaturation in the human body determines the possibility of drug bioequivalence in vivo. Increasing the dosage without authorization may intensify the nucleation of the drug in the human body, causing it to precipitate faster and be absorbed worse. The pH shift model simulates the process of drug movement in the human GI, providing an inexpensive and accurate human simulation solution in an in vitro manner. It offers a repeatable and high-throughput tool for formulators to screen appropriate drug dosages and to select excipients that inhibit the supersaturated precipitation effect of drugs.
The Accurate algorithm built into NCE DP enables the spectrometer to measure the concentration of drugs in a turbid state. The test results can be directly calculated through the built-in algorithm, allowing the effect of excipients to be known in real time without further processing of the sample. This will significantly increase the speed of drug development for pharmacists and make it easier for you to screen excipients.

