Excipients

Excipients are an important part of the drug, and some special excipients will have an effect on the dissolution/permeation of the drug, so the effect of excipients needs to be considered in the drug development process.
Get Free Quote
Experimental parameters
| Experimental conditions | Pure API (control experiment) | Reference preparation | Text preparation |
| Bionic Membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane |
| Supply side | pH 6.5 buffer | pH 6.5 buffer | pH 6.5 buffer |
| Receiving side | pH 7.4 buffer | pH 7.4 buffer | pH 7.4 buffer |
| Sample conditions | Pure API without excipients | Reference preparation with excipients | Text preparation with substitute excipients |
Experimental result
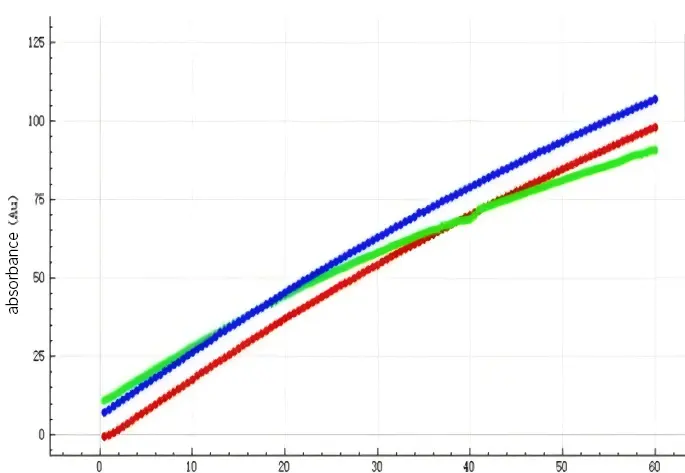
The case we share this time is an acidic drug with API of BCS2 class, as an innovative formulation, some of its excipients are not available for purchase in China. So, the consistency of the four dissolution curves was achieved by using the method of excipient substitution, however, in the process of BE, it was found that the effect of the sample was inconsistent with that of the reference. It is now necessary to understand through experiments, why the dissolution curves are consistent but the BE is not.
Conclusion
Based on the permeation results of NCE DP, the curves of the reference preparation and API were parallel, indicating that the excipients used in the reference had no effect on the permeability of the drug at all, and the difference between them was only in the concentration of the drug. While the permeation curves in the sample preparation showed a decrease in the permeation rate compared to the pure API, indicating that the excipients used in the sample preparation had an effect on the permeability of the drug, which had no significant difference in the first period of the test (0-20 min), and began to show a significant difference after 20 min.
According to the results of the dissolution instrumental test by the flow-through cell method, it was shown that there was indeed a slight difference between them in terms of dissolution, with the dissolution rate of the reference being higher than that of the sample in the middle stage of dissolution.
It can be seen that the clinical effects of sample are quite different from the reference , and substitution of excipients does not make the drug effects exactly the same as the reference.

