In Vitro Release
,散落在白色表面上.-1761719697785.webp)
In vitro release refers to the experimental measurement of the rate of drug release from a formulation under conditions that mimic the in vivo environment (e.g., temperature, pH, agitation rate, etc.). It is a method used to monitor the manufacturing process and quality control of a product, and can help to develop reasonable in vitro drug release metrics.
Get Free Quote
Experimental parameters
| Dissolution Apparatus | RT700 |
| Cell | Large Cell (22.6mm) |
Experimental result
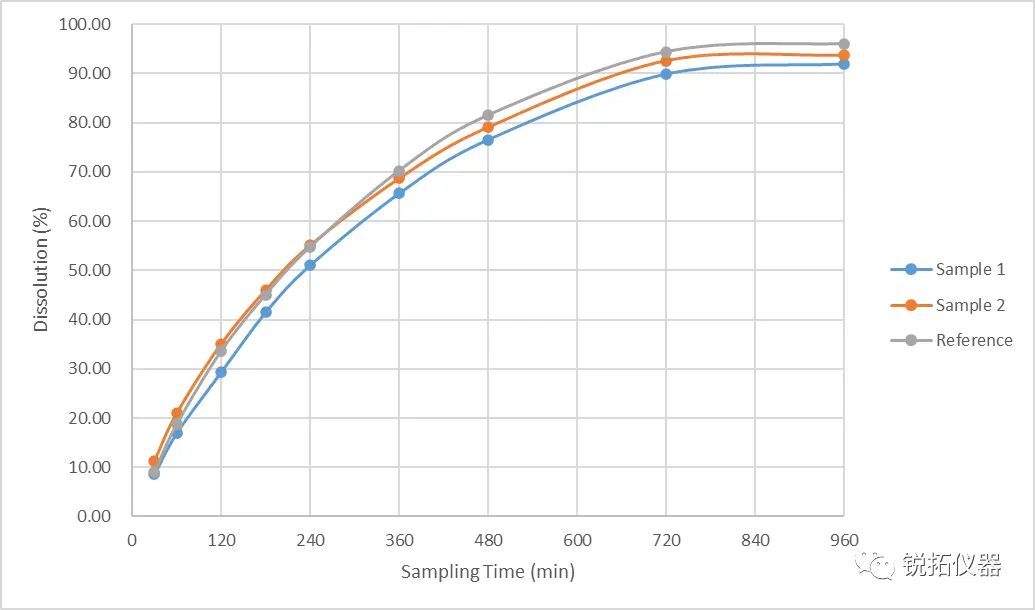
Preliminary test results - no correlation with in vivo pharmacokinetic data According to the BE data of the self-research sample 1 (Sample 1) and the self-research sample 2 (Sample 2): the dosing time profile of the self-research sample 1 is above the dosing time profile of the reference preparation (Reference), and its C max and AUC are significantly higher than those of the reference preparation; the dosing time profile of the self-research sample 2 is below the dosing time profile of the reference preparation, and its C max and AUC are lower than those of the reference preparation. The C max and AUC of the self-research sample 2 were lower than those of the reference preparation. However, there was no correlation between the preliminary in vitro release results and the in vivo pharmacokinetic data: (1) The release rate and cumulative dissolution of the self-research sample 2 were slightly higher than that of the self-research sample 1. (2) The in vitro release results of the reference formulation and Self-Investigated Sample 2 were close enough that it was not possible to effectively differentiate between them. In addition, overall the in vitro release profiles of all three samples were close, and the method differentiation power was less than ideal. The preliminary test results are shown above:
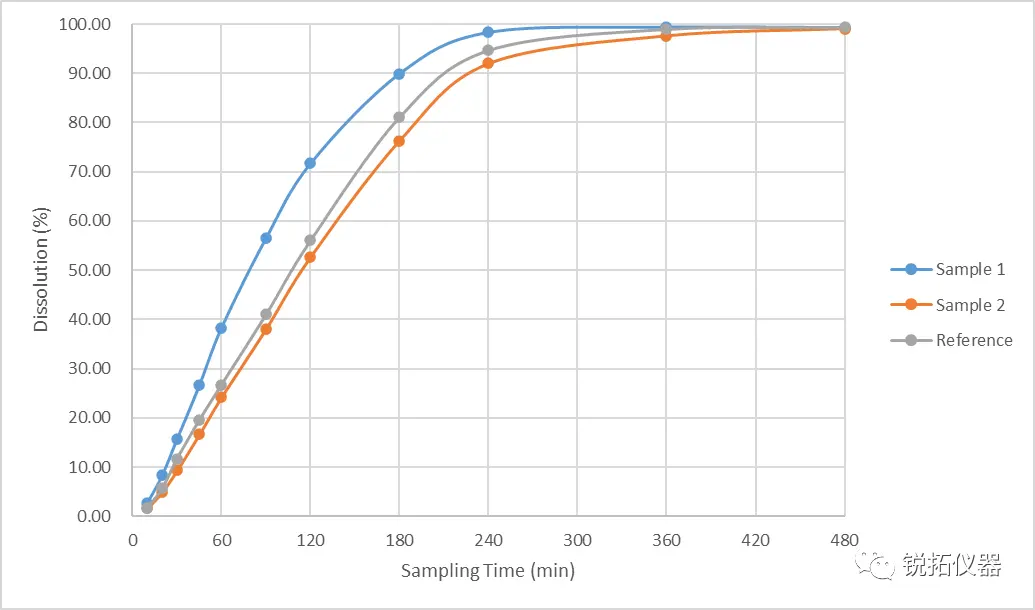
Through in-depth analysis of the in vitro release phenomena, test results and experimental parameters of the samples, we deduced that some of the experimental parameters of the flow cell method limited the real release of the samples. Further, we redesigned the experimental method based on the release principle, dosage form and process characteristics of the samples. Test results with correlation to in vivo pharmacokinetic data The results of the redesigned in vitro releasability test by the flow cell method were in line with the intended goals of our experimental design: The release rate of self-research sample 1 was faster than the reference formulation, and the release rate of self-research sample 2 was slower than the reference formulation, and there was a correlation between the test results and the in vivo pharmacokinetic data. The test results are shown in the figure above:
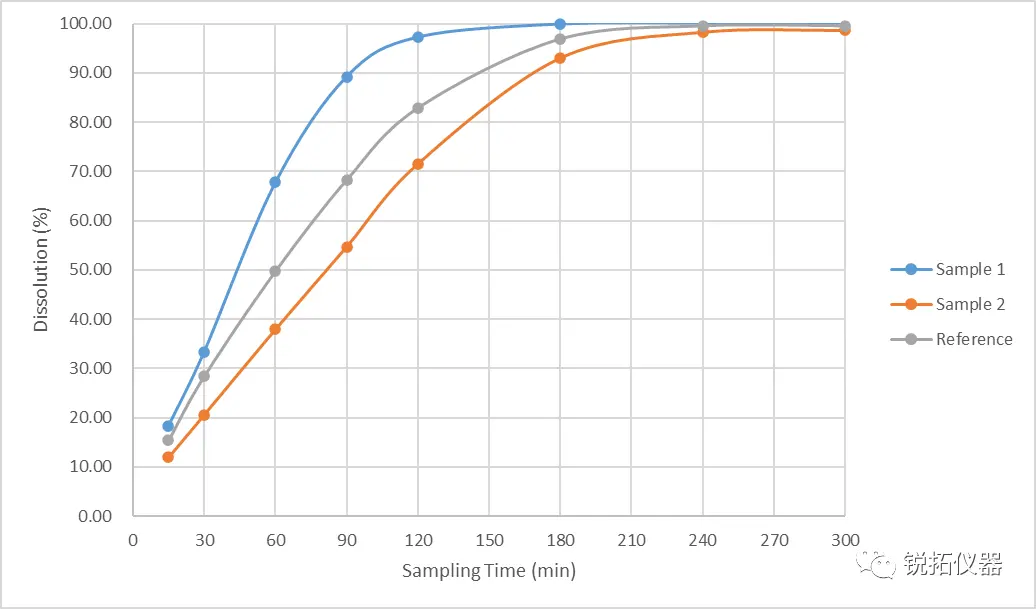
Based on the in vitro release profiles, we also found a small regret about the parameters of this method: although it can reflect the difference in release between self-developed Sample 2 and the reference formulation, the difference between the two is relatively small and not as significant as that between self-developed Sample 1 and the reference formulation. Reviewing the BE data of Self-Study Sample 1 and Self-Study Sample 2, the degree of difference between the in vivo pharmacokinetic data of Self-Study Sample 2 and the reference formulation is indeed smaller than that of Self-Study Sample 1 and the reference formulation. This further confirms that the new method is feasible and worth continuing to optimize for better discriminatory power. For better discriminatory power In order to better differentiate the in vitro release difference between the self-developed sample 2 and the reference formulation, we readjusted the test parameters and examined the effects of key parameters on the test results using control variables, such as: pH of the dissolution medium, surfactant concentration, flow rate, the amount of glass beads filled at the bottom of the flow cell, and the position of the samples placed. After the above test parameters were optimized, the in vitro release degree results were better differentiated and still correlated with the in vivo pharmacokinetic data. The test results are shown above:
Results & Discussion
After redesigning the original flow cell dissolution method and optimizing the key parameters, the in vitro release results correlated with the in vivo pharmacokinetic data with good discriminatory power.
The development of the method was in line with the expected goals of the experimental design thanks to the thorough technical communication with the client. The in vivo pharmacokinetic data, the sample preparation process, the design differences between different self-developed samples and other key information provided by the customer made the whole method development and optimization process more targeted, and made the final in vitro release test method and results have scientific logic and practical significance.
The results of this test also reconfirmed the potential of the flow cell method to predict the pharmacokinetic characteristics of drugs in vivo, which can greatly facilitate the research, screening and optimization of the formulation process and make drug development more efficient.

