Vaginal tablets

Vaginal tablets are tablets that are placed in the vagina for use. It is characterized by easy dissolution, or melting, disintegration and release of drugs in the vagina. They are absorbed through the vaginal mucosa into the local or systemic blood circulation and are often used in the treatment of vaginitis and other clinical diseases. They can be divided into ordinary vaginal tablets, double-layer vaginal tablets, vaginal effervescent tablets, vaginal slow-release tablets, and bio-adhesive vaginal tablets (the latter three of which are new vaginal dosage forms, with the main research focusing on how to improve the adhesion of the preparations or give the preparations a slow and controlled release performance).
Get Free Quote
Experimental parameters
| Main analyzing instrument | Raytor RT700 flow cell dissolution system |
| Dissolution device | Flow-through cell (Standard cell, 22.6mm inner diameter) |
| Temperature | 37℃± 0.5℃ |
| Flow rate | 16mL/min |
| Pulsation mode | Pulsation-free mode |
Experimental results
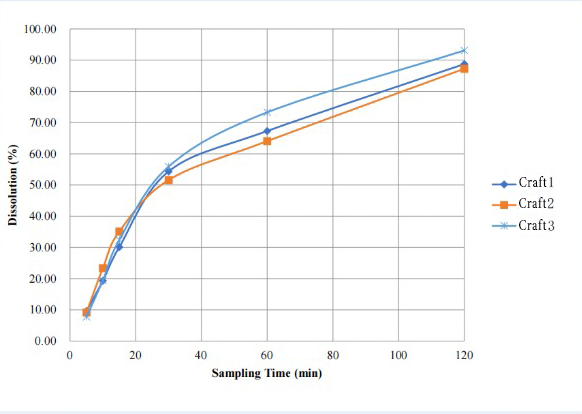
In this test, we simultaneously conducted in vitro release tests on three batches of clotrimazole vaginal tablets, and the final test results showed that the experimental parameters of the cell method successfully differentiated the in vitro release behavior of clotrimazole tablets with three different prescription processes. It provides a valuable reference for the subsequent adjustment of the prescription process. And there was no blockage in the cell during the whole experimental process (at 120 min, the dissolution amount of the samples from the three batches was >85%).

5 min

60 min

120 min
Summary:
Vaginal drug delivery system has unique use and therapeutic advantages, and has a good development prospect. Clotrimazole vaginal tablets are widely used in clinical practice and are in great demand.CFDA listed it as one of the varieties of the National Drug Quality Evaluative Sampling and Testing Program as early as 2016, and required testing organizations to evaluate its current quality standards and the quality of the product.
Therefore, in order to ensure that its quality is controllable and its efficacy is reliable, and to ensure the safety of the clinical use of this drug. In the process of carrying out the research and development of generic drugs of this variety, researchers should choose the correct equipment and experimental parameters according to the development of various new in vitro drug dissolution test devices and biological dissolution methods in recent years.

