Semi-solid formulations - Creams
Semi-solid formulations, owing to their swallowability, ease of use, and low side effects, have gradually gained popularity among many company drug developers due to aging populations. Today, semi-solid formulations can be broadly categorized into ointments, creams, pastes, and gels.
Get Free Quote
Experimental parameters
| Test system | RT800 Automated Transdermal Diffusion System |
| Diffusion cell | Standard Franz vertical diffusion cell with an inner aperture of 15mm |
| Sample loading volume | approximately 350mg |
| Temperature | 32℃± 0.5℃ |
| Sampling time | 0.5、1、2、4、6、10、16、24、36、48、72 hours |
| HPLC detection parameters (for the obtained samples, the drug concentration in the samples needs to be analyzed by high performance liquid chromatography) | |
| Detector | UV 241nm |
| Flow rate | 1.2mL/min |
| Mobile phase | Acetonitrile / aqueous solution |
| Chromatographic column | ZORBAX SB-CN |
Experimental results
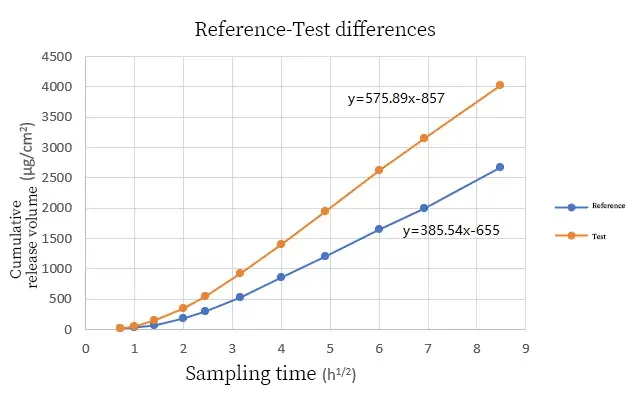
Figure 1 Investigation of differences among samples: The release rate of the drug in the cream was represented by the square root graph of the cumulative drug release amount (μg/cm2) and time (t), where the k value represents the drug release rate in the formula. The results are shown in Figure 1. The average drug release rate of the reference sample is 385.54, and that of the test sample is 575.89, indicating that the drug release of the test sample is faster than that of the reference sample. Through the determination of the cumulative drug release amount, the maximum value of the test sample is significantly greater than that of the reference sample. This also proves that the difference in drug release rates among samples leads to different drug release amounts within the same period of time.
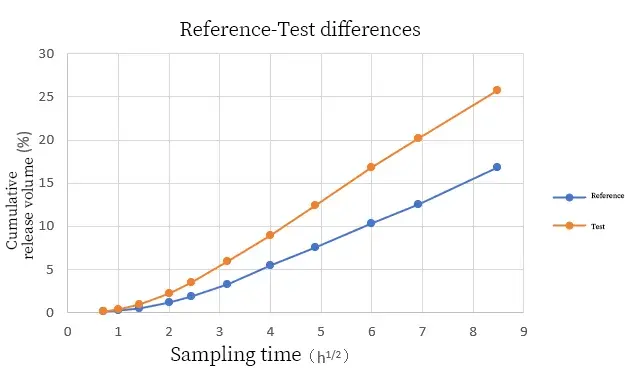
In addition to the cumulative drug release amount, the cumulative release rate of the drug (Figure 2) can also indicate the difference in the release of drug molecules from the formulation between the two samples. Through IVRT tests on the two samples, the test samples showed significant differences compared with the reference samples in terms of cumulative drug release amount, cumulative drug release rate and cumulative release rate.
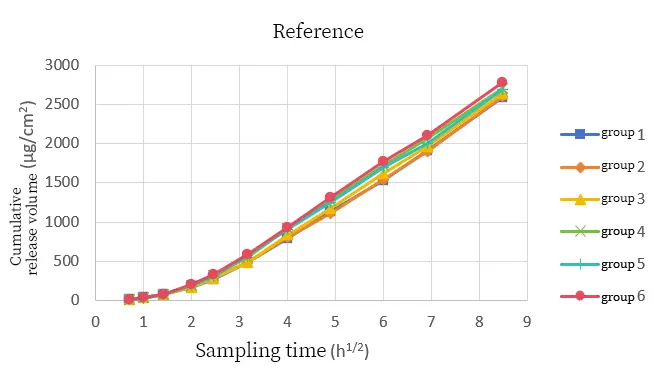
To ensure that the test results can accurately reflect the differences among samples, it is necessary to examine the testing methods. The relevant guidelines for the validation of analytical methods in the Chinese Pharmacopoeia state that the purpose of the validation of analytical methods is to prove that the established method is suitable for the corresponding testing requirements. The key indicator for verification is repeatability. In this case, we conducted six parallel tests on each of the two samples under the same test conditions. The test results are shown in Figures 3 and 4 as follows:
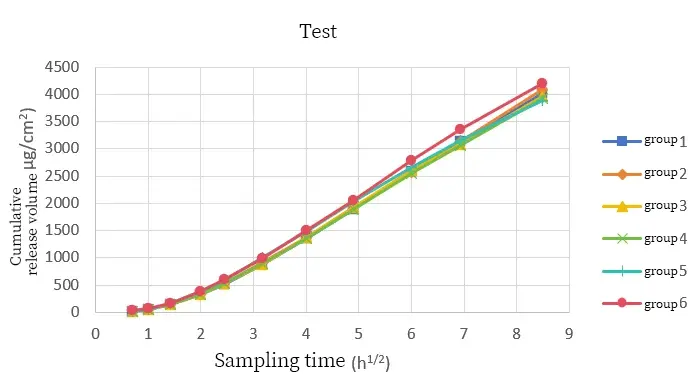
The cumulative drug release curves of the two samples had a good overlap under six parallel tests. The relative standard deviation of the cumulative drug release at the sampling endpoint of the reference sample was 2.69%, and that of the test sample was 2.85%. After six tests on different samples under the same method, the relative standard deviations obtained by the instrument were not much different, indicating that the instrument has good testing stability.
Conclusion
Semi-solid dosage forms, as a way of drug formulation innovation, have gradually attracted the attention of researchers. However, semi-solid preparations, due to their complex processes and relatively difficult method development, pose certain challenges to researchers in the development of preparations. For this reason, researchers need to use appropriate instruments and equipment to assist themselves in solving a series of problems during the development of semi-solid preparations.
From the above case sharing, it can be seen that the automatic sampling transdermal diffusion system can provide strong and favorable support for the in vitro release research of semi-solid preparations. Moreover, under reasonable and appropriate release conditions, it can compare the cumulative drug release amount, cumulative drug release rate and cumulative release rate among samples, significantly distinguishing the differences among samples. Moreover, the testing method can meet the requirements for method repeatability and discriminability stipulated in the relevant guiding principles.···

