How to Select the Best Equipment for Transdermal Diffusion Testing is a practical question with real stakes. The Best Equipment For Transdermal Diffusion Testing protects data, time, and compliance. Many labs still buy on price or reputation. They overlook quiet factors: synchronized sampling, tubing length, bubble control, audit trails. One weak link can bend release and permeation curves. One shortcut invites repeat runs and tough audit questions. What if you could screen vendors in minutes and avoid costly surprises? In the next section, we reveal the signals to trust - and the red flags hiding in plain sight.

How to Select the Best Equipment for Transdermal Diffusion Testing
Selecting a platform is more than buying hardware. It is a decision about risk, throughput, and data integrity. Begin by aligning your method with the rules that govern your work. For transdermal patches and semisolid products, the equipment must operate within USP <1724> performance tests and EP 9.0 <2.9.4>. If your setup cannot meet these frameworks natively, you will spend time on workarounds, extra documentation, and avoidable deviations.
- Start with standards, not shortcuts
Compliance should be the default behavior, not a side project. Look for Franz diffusion cells consistent with the style described in USP <1724>. Pair those cells with software that records who did what and when, preserves method versions, and secures user access. When the basics are embedded, your team can focus on science rather than paperwork.
- Minimize human variance at the source
The biggest hidden variable is the operator. Manual sampling drifts. Timing slips by seconds. Long tubing traps residue. Bubbles shift effective surface area. The Best Equipment For Transdermal Diffusion Testing avoids these traps with synchronized sampling, short fluid paths, and clear visual controls that make correct technique obvious. These details reduce spread in your permeation curves and improve study repeatability.
- Choose one platform for IVRT and IVPT
Running in vitro release tests (IVRT) and in vitro permeation tests (IVPT) on the same system lowers training time and validation overhead. It also simplifies method transfer. When your team can move from release to permeation with minimal changeover, you cut delays and reduce the chance of errors.
Why RAYTOR Builds the Best Equipment for Transdermal Diffusion Testing
We design our automatic sampling transdermal diffusion system to remove timing bias and operator fatigue. The system performs cleaning, sampling, and refilling automatically, run after run. It supports IVRT and IVPT within USP <1724> and EP 9.0 <2.9.4>, so method alignment is direct and defensible.
- Hardware choices that matter in daily use
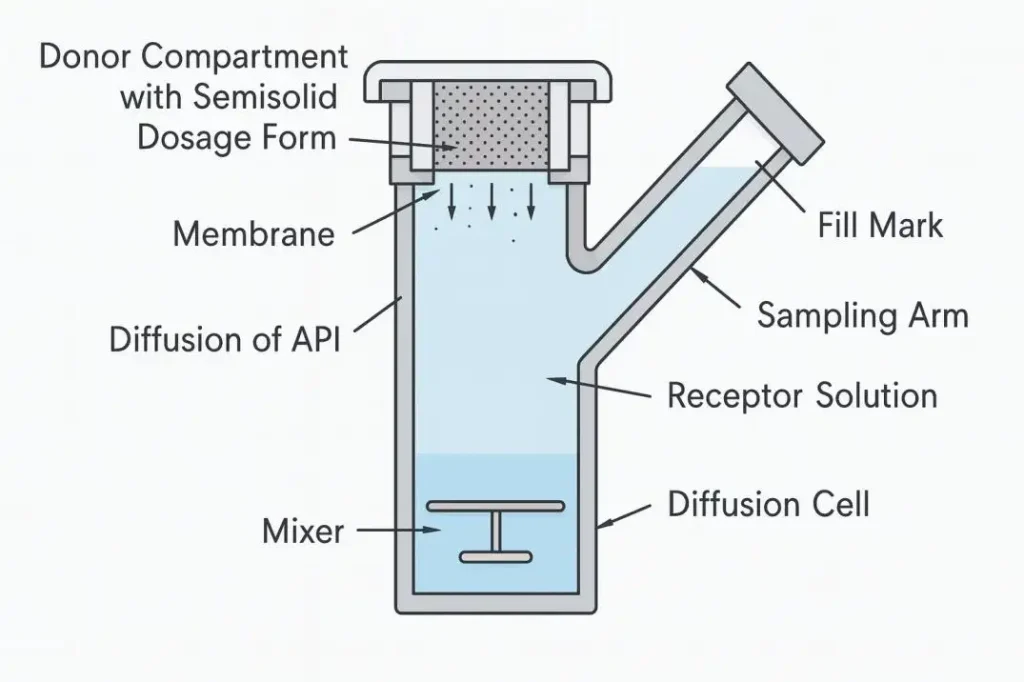
Short pathways are not a cosmetic feature; they lower carryover risk. Our shorter pipeline design effectively reduces sample residue. The open operating space gives technicians room to work, while a clearly marked filling point on each diffusion cell guides medium level setting after bubbles are removed. Diffusion cells lift out and lock back in quickly, so checks and bubble discharge take minutes, not cycles.
- Synchronized sampling at scale
Sampling precision defines data quality. Our platform offers seven synchronized channels. Cells in the same group sample at the same moment, which keeps your concentration-time profiles aligned. You can run two independent groups in parallel without cross-interference. A full set supports 6 + 1 diffusion cells, so you can dedicate a blank to exclude external interference and keep baselines clean.
- Data integrity by default
Good data must tell a complete story. Our operating system provides a full audit trail and an SQL database. Labs can store no fewer than 200 test methods and no fewer than 100 user accounts. Method governance and user management scale with your team, not against it. For many customers, this is the line between data you can publish and data you must explain away.
A Practical Checklist and Your Next Step
When you compare vendors, keep the conversation grounded in outcomes: clean kinetics, reproducibility, and smooth inspections. Use the following quick screen to separate promises from proof.
• Standards fit: Native alignment with USP <1724> and EP 9.0 <2.9.4> for patches, IVRT, and IVPT.
• Sampling control: Seven-channel synchronized automatic sampling with two independent groups for parallel runs.
• Low-residue flow path: Short pipelines to limit carryover and protect concentration curves.
• Operator guidance: Marked filling point, open workspace, and easy cell removal to discharge bubbles correctly.
• Capacity and controls: 6 + 1 diffusion cells per set, enabling a blank to remove outside interference.
• Data integrity: Audit trail + SQL database, storage for ≥ 200 methods and ≥ 100 accounts.
• Throughput and flexibility: Automated cleaning, sampling, and refilling to reduce manual steps and timing drift.
✅ What this means for your lab
In practice, these capabilities cut down on repeated runs and arguments over outliers. They shorten investigations, because your audit trail shows the exact sequence of actions. They also speed onboarding. New analysts follow visual cues - the filling point mark, the open space around each cell - and the system handles the hardest timing tasks for them. The result is method performance that looks the same on Monday morning and Friday afternoon.
✅ How RAYTOR supports implementation
We help teams translate protocol language into day-to-day operation. That starts with mapping your acceptance criteria to system controls: sampling intervals, synchronized group triggers, and refill routines. Then we tune your workflow so IVRT and IVPT can run on the same bench with minimal reconfiguration. Because the software retains methods and user roles, you can enforce good practice without adding administrative weight.
Evaluate Before You Invest
If your priority is reliable kinetics and audit-ready documentation, the Best Equipment For Transdermal Diffusion Testing should make those outcomes routine rather than exceptional. Our approach is to remove bias at every step - hardware, sampling, and data capture - so the science speaks clearly.
Call to Action: Ready to see how RAYTOR fits your IVRT/IVPT roadmap? Contact us for a focused demo or method review. We'll walk you through synchronized sampling, compliant diffusion cell design, and audit-trail workflows - so you can move from evaluation to confident results with speed.