-

Intelligent Leadership, Precision and Efficiency: Raytor Instruments Successfully Finishes 2025 National Pharmaceutical R&D Innovation Conference
-
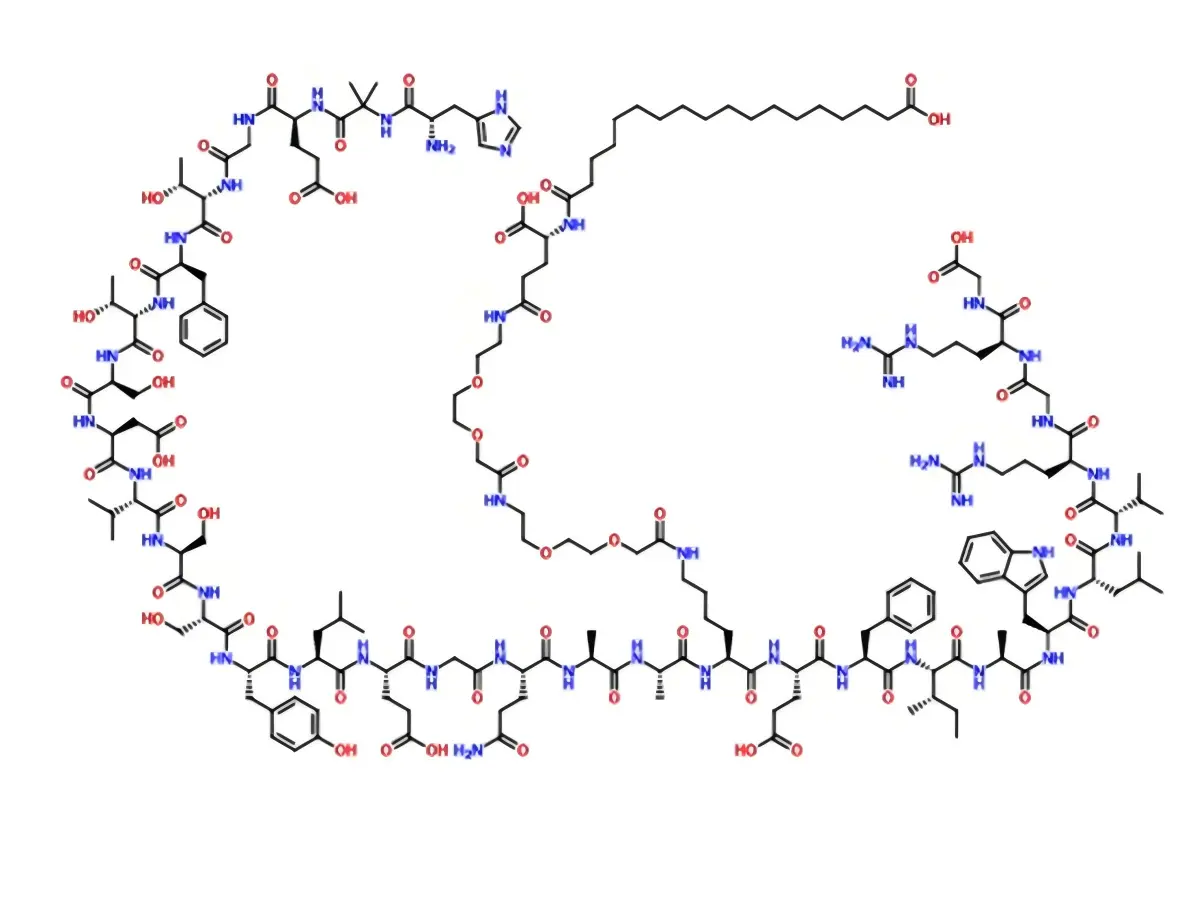
How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
-

What Is Precision Laboratory Automation and Why It Matters
Dissolution Tester For Quality Control is more than lab hardware; it is a safety net for data, timelines, and audits. When results drift, reputations do too. Your team needs accuracy you can prove, not just promise. A reliable system keeps temperatures stable, dosing synchronized, and sampling clean. It reduces repeats and stops small errors from becoming big deviations. Yet most labs still accept hidden risks in daily runs. Why? Because the problems are subtle - and expensive. In the next section, we'll reveal the quiet failures you're likely missing, and how to fix them before your next batch.

Reliability That Safeguards Compliance And Throughput
Auditors care about two things above all: control and proof. A reliable Dissolution Tester For Quality Control must deliver both under real lab conditions, not just in demo runs.
Raytor's system is built around international standards - ChP (0931), USP (711/724), and EP (2.9.3/4) - so methods map cleanly to regulations without extra validation cycles. A high-capacity, dual-module, auto-sampling layout supports parallel work: two independent dissolution tests can run at the same time with different devices, speeds, solvents, and sampling schedules. That flexibility cuts queues and keeps projects moving when timelines are tight.
Precision shows up in the small things that cause big headaches. Vessel-level temperature monitoring with independent probes provides real-time visibility, minimizing excursions that trigger costly investigations. Covered dissolving cups and automatic centering stabilize hydrodynamics and speed setup. A one-piece paddle shaft reduces alignment drift and mechanical wear, keeping results consistent across batches and analysts.
- Why It Matters In Practice?
• Fewer repeats caused by thermal drift or misalignment
• Clear traceability that shortens audit discussions
• Higher sample capacity without adding instruments
- Solving The Sampling And Filtration Pain Point
Many labs still swap filters by hand and leave a sampling needle in the vessel throughout the run. Both practices disturb flow patterns and add variability. Raytor's intelligent online filtering supports storage and automatic replacement of secondary filters, while a non-resident sampling needle minimizes turbulence. Analysts intervene less, data trend tighter, and downstream analytics see fewer particulates.

Intelligent Automation That Reduces Risk And Hidden Cost
Hidden cost in QC comes from time sinks: re-calibrations after method changes, dose timing drift, mid-run adjustments, and unplanned pauses. A dependable Dissolution Tester For Quality Control should make those problems rare.
- Automation Built For Real Methods
Automatic synchronized dosing eliminates the time-of-dose differences that skew early time points, and every dose event is tracked for traceability. The one-piece paddle shaft allows quick method switching without re-calibrating height when shifting between basket and paddle methods. During a run, method rules can permit changes to dissolution medium volume and rotational speed - no stop-and-start; no guesswork.
- Autosampler That Works The Way You Do
An advanced autosampler manages online secondary filter replacement and automatically positions the sampling needle based on your method and solvent volume. This reduces clogs and lowers background particulates, which in turn protects HPLC/UV systems and decreases the chance of repeat testing.
- Operational Wins You'll Notice Fast:
• Less hands-on time thanks to automatic filter handling
• Fewer deviations linked to dose timing errors
• Faster changeovers between products or strengths
• Live display of each vessel's temperature for proactive control
Consistency Across Forms And Batches
Quality teams need one platform for many tasks. Basket and paddle methods are the backbone of pharmacopoeial guidance, and both must run reliably. A modern Dissolution Tester For Quality Control should support oral tablets, transdermal patches, semi-solid preparations, injections, and intrinsic dissolution rate (IDR) studies for APIs - without a maze of setup changes.

In daily use, covered cups and automatic centering simplify installation. Real-time temperature monitoring builds confidence that all vessels follow the method, not just an average. Parallel, independent test capability lets you compare formulations, suppliers, or process tweaks side by side under controlled conditions. The effect is felt in shorter investigations, cleaner trending, and more decisive go/no-go calls.
- How Consistency Becomes A Competitive Edge:
• Stable hydrodynamics via non-resident sampling and synchronized dosing
• High-precision, vessel-level temperature control
• Dual, independent runs for A/B comparisons within one shift
• Method integrity even when adjusting medium volume or RPM within validated limits
Why Raytor
Raytor's mission - research, development, production, sales, and service - shapes every design decision. We bring precision mechanics together with practical automation: intelligent filtering, synchronized dosing, automatic needle positioning, and granular temperature oversight. The goal is simple: reduce variability at the source so your scientists spend time interpreting data, not troubleshooting hardware.
- What Makes The System Practical Day To Day?
• Dual-Mode Dissolution System: Run two methods at once to cut queues and accelerate stability or reformulation work.
• Intelligent Online Filtering: Store and automatically replace secondary filters to keep baselines clean.
• Real-Time Monitoring: Independent probes display solvent temperature for each vessel.
• Automatic Synchronized Dosing: Remove timing differences and record dose events for full traceability.
• Automatic Needle Positioning: Match needle depth to method and solvent volume to shrink variability.
• Covered Cups And Auto Centering: Faster setup and more durable components for everyday reliability.
Call To Action: See Reliability In Action
If you are ready to tighten compliance, reduce repeats, and speed release decisions, talk to Raytor. Our team can review your SOPs, map risks, and configure a Dissolution Tester For Quality Control that matches your methods and throughput targets. We also support method transfer and validation, so your lab moves from pilot to routine testing with confidence. Start the conversation today. With Raytor, reliability is engineered, not assumed - and your QC operation gains a dissolution platform built for today's regulatory scrutiny and tomorrow's pipeline growth.
