How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
2025-10-22
Background.
Peptide drugs represent a large percentage of the pharmaceutical market, with global sales of over $70 billion in 2019, more than tripling from 2013.According to relevant data, the top 200 drugs in sales in 2019, include 10 non-insulin peptide drugs. Interestingly, the top three peptide drug sales are all GLP-1 analogs used to treat T2DM, with Trulicity (dulaglutide, subcutaneous) ranked 19th with $4.39 billion in retail sales, Victoza (liraglutide, subcutaneous) ranked 32nd with $3.29 billion in sales, and Rybelsus (somatostatin. oral) ranked 83rd with $1.68 billion in sales. Although peptide drugs have so many advantages, due to the weak membrane permeability of peptides. The membrane permeability of peptide drugs depends on a number of factors, including peptide length and amino acid composition. Peptides are usually unable to cross cell membranes to target intracellular targets, thus limiting their use in drug development. To date, the most successful and advanced clinical strategy has been the co-formulation of orally administered peptides with excipients that transiently increase intestinal membrane permeability. However, these studies tend to use animals as research subjects and there is a lack of inexpensive, reproducible experimental methods to study the permeability of peptide drugs in the intestinal membrane.
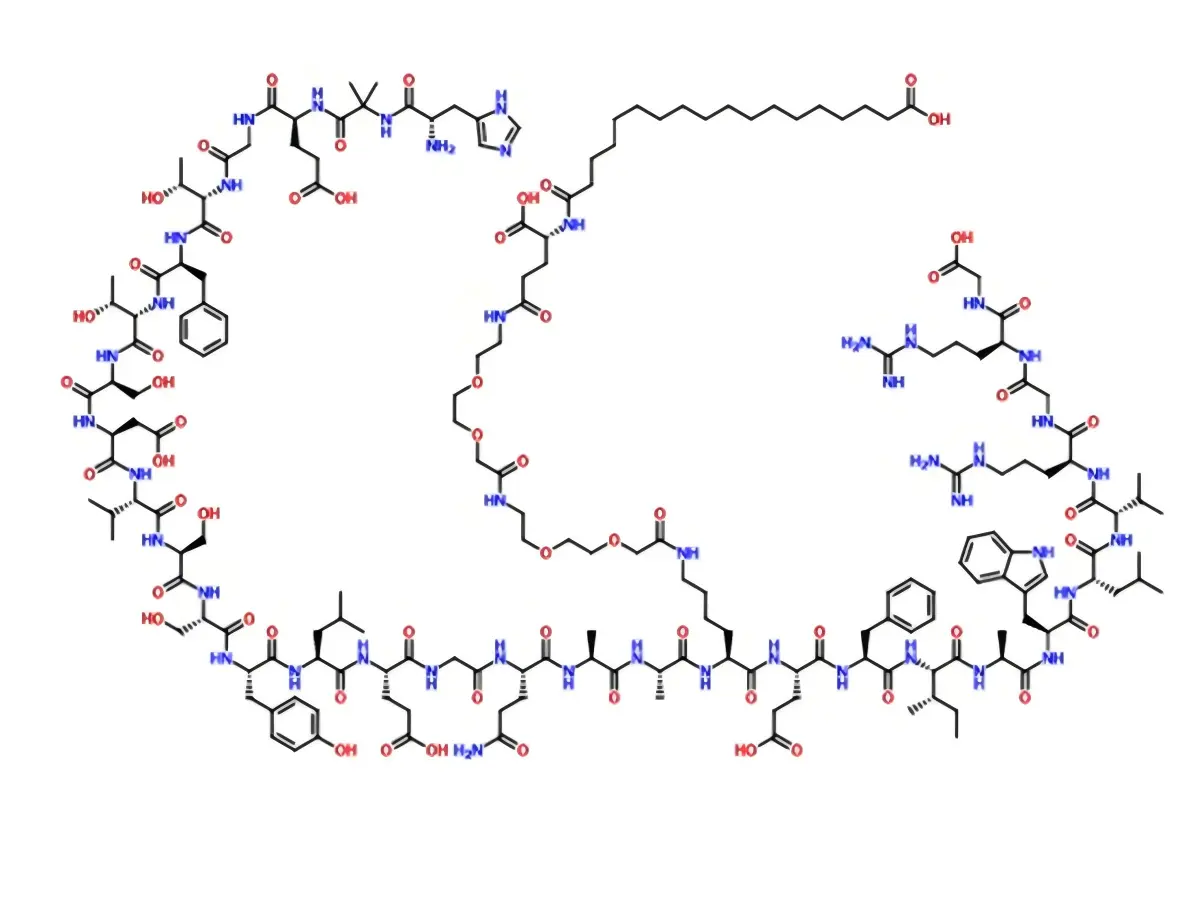
Semaglutide is a novel long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA), which is structurally similar to natural human glucagon-like peptide-1 (GLP-1), with up to 94% amino acid sequence homology. and it is used for blood glucose control in adult patients with type 2 diabetes mellitus.
| Experimental condition | Control experiment | Test | Reference |
| Bionic membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane | Raytor Bio-Flux Membrane |
| Supply side | pH 6.5 buffer | pH 6.5 buffer | pH 6.5 buffer |
| Receive side | pH 7.4 buffer | pH 7.4 buffer | pH 7.4 buffer |
| API | Semaglutide | Semaglutide | Semaglutide |
| Excipient | No excipients added | Contains a penetration enhancer | Novo Nordisk Original Research Excipients |
The permeability model in Raytor NCE DP was used to study the compatibility permeability of excipients & api (with PAMPA method)
Reference: The reference of semaglutide is a new generation GLP-1 (glucagon-like peptide-1) analog developed by Novo Nordisk.
Test sample: The addition of other excipient is used for permeability comparison with the reference.
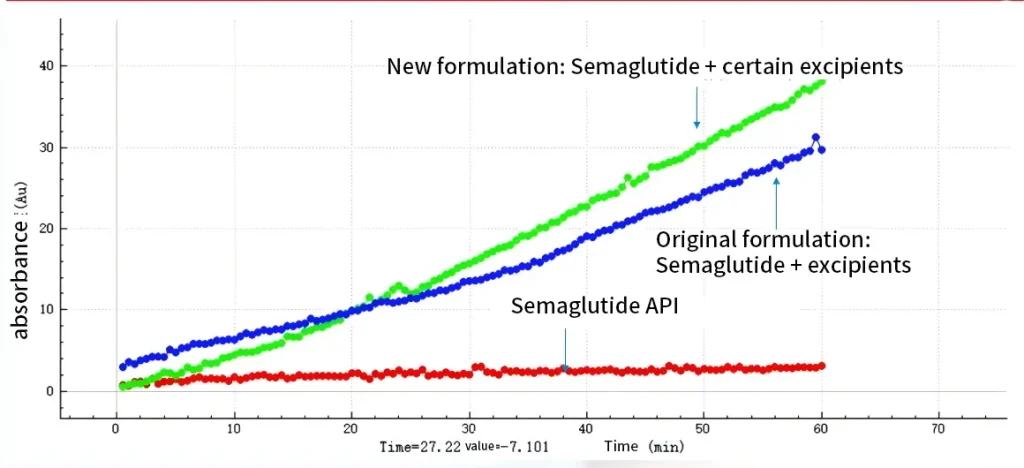
Conclusion:
The results of the PAMPA experiments in the NCE DP suggest that semaglutide with some kind of permeation enhancer can significantly enhance its absorption (much greater permeation than a pure API) and perhaps have a better absorption enhancement effect than the original formulation.
Discussion:
Peptide drugs are difficult to make into oral peptide drugs because of their poor absorption ability. but if you can find suitable excipients to enhance their permeability, the peptide drugs made into innovative formulations are also very valuable research and development programs. With NCE DP's PAMPA permeation model, the permeation ability of various excipients with peptide drugs can be studied in a high-throughput and high reproducibility, making your peptide drug prescription research easier.