How To Test Transdermal Drug Absorption is the question every clinical team faces when patches, gels, ointments, or creams move from bench to bedside. At Raytor, we build instruments that make this work reproducible, audit-ready, and fast. In this article, we explain How To Test Transdermal Drug Absorption In Clinical Research with a practical, compliant workflow - and show how Raytor's automated diffusion system supports IVRT and IVPT from day one.
Why Testing Transdermal Absorption Is Hard in Clinics
Clinical environments are busy and variable. That creates risk for diffusion studies. Small temperature drifts, pipetting delays, or sample residue can skew permeation curves and mislead dose decisions. Investigators also face documentation pressure: protocols must map to pharmacopeial chapters, and every analytical step must be traceable.
At Raytor, we see three recurring blockers. First, time - manual cleaning, refilling, and staggered sampling add hours and introduce timing bias. Second, residue - long sampling lines trap analyte and flatten real signals. Third, compliance - teams need clear alignment with European Pharmacopoeia (EP 9.0) 2.9.4 for transdermal patches and USP <1724> for semisolid performance tests, especially for IVRT and IVPT. When those three issues converge, reviewers lose confidence and studies repeat. We designed our automated diffusion platform to remove that friction.
• Standards-Compliant Methods (IVRT & IVPT)
Raytor's system supports in vitro release tests (IVRT) and in vitro permeation tests (IVPT) under USP <1724> and EP 9.0 2.9.4. The diffusion cell style follows the 5th-type description in USP <1724>, and the open working space makes loading and sampling straightforward. This ensures your protocol language aligns with the monographs - from receptor medium preparation to sampling cadence - and helps translate bench data into clinical evidence. For teams searching a Franz diffusion cell IVPT protocol, we provide templates, timing guidance, and instrument-level safeguards to reduce deviation.
• Automation That Improves Data Integrity
Automation is not just convenience; it improves truthfulness of data. Our platform performs complex cleaning, synchronized sampling, and automatic refilling to keep timing consistent and human contact minimal. Shorter pipeline design reduces hold-up volume, cutting the risk of residue and carryover. Simultaneous sampling across 7 channels eliminates inter-cell timing bias, while two independent groups let you run test and blank (or method development and clinical support) in parallel without interference. An embedded audit trail with an SQL database records actions, users, and methods, supporting audit readiness during and after trials.
How To Test Transdermal Drug Absorption With Raytor
Start with the clinical question - exposure target or comparative bioavailability - and translate it into a diffusion plan. Choose IVRT for release characterization or IVPT for barrier crossing. The Raytor system's open operating space speeds mounting; the filling point mark helps you remove bubbles and confirm the receptor phase is at level, preventing future bubble formation and artifacts.
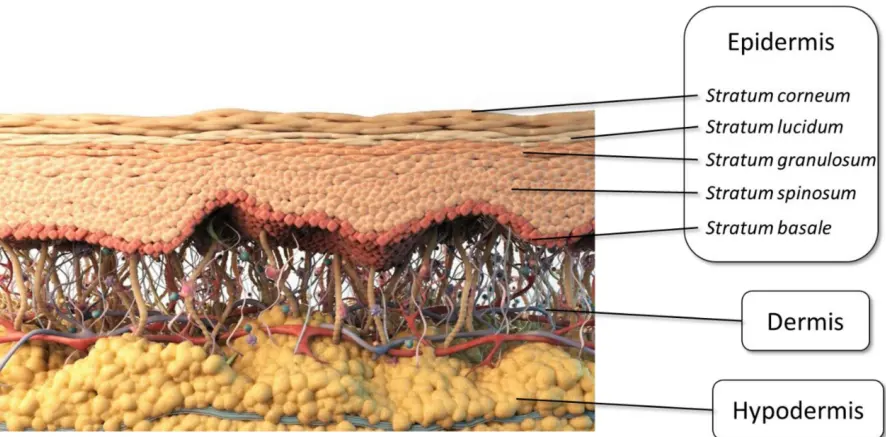
Set temperature (room temperature to 45 °C, error ≤ ±0.5 °C) and agitation (200–900 rpm, error < ±10%). Select diffusion cell volumes (10, 15, 20, 25, 30, or 40 mL) to fit your matrix and LOQ. Define up to 24 sampling events with volumes from 0.2-1.5 mL; maximum programmable runtime is 9,999 minutes, so extended permeation is covered. For in vitro permeation test for creams and gels, run one group as blank to subtract matrix interference while the second group captures active permeation. Because sampling is synchronized, your timepoints remain truly comparable.
The operating system can store no fewer than 200 test methods and 100 user accounts, keeping multi-site teams aligned. Manual bubble exhaust is available before locking the run, and cells are easy to remove and reinstall for checks. These small usability details add up to smoother clinical support: fewer restarts, cleaner chromatograms, and tighter confidence intervals.
✅ Where This Matters Most
Patches, gels, creams, and ointments behave differently under clinical constraints. Patches demand consistency with EP 2.9.4; semisolids rely on USP <1724>. In both cases, reviewers expect method suitability, stable temperature, precise sampling, and reproducible flux profiles. By combining compliant cells, synchronized timing, and short lines, Raytor helps you reach those endpoints faster and with fewer queries from QA.

✅ Key Specifications & Benefits
• Compliance built-in: Aligns with EP 9.0 2.9.4 (transdermal patches) and USP <1724> (semisolid performance tests: IVRT/IVPT).
•Channels & groups: 7 synchronized sampling channels per group; two independent groups for parallel or blank runs; diffusion cell positions 7×2.
•Diffusion cells: USP-described 5th-type compliant style; easy on/off for bubble checks; clear injection/filling point marks.
•Sampling control: Up to 24 sampling times; 0.2–1.5 mL per draw; simultaneous across a group to prevent timing bias.
•Thermal & mixing: Room temp–45 °C with ≤±0.5 °C error; 200–900 rpm with <±10% error; dry heating for stable operation.
•Data integrity: Full audit trail, SQL database, method library (≥200 methods) and user management (≥100 accounts).
•Low residue design: Shorter pipelines reduce sample hold-up and carryover, improving accuracy and cleaning efficiency.
•Applications: Creams, ointments, patches, gels - from method development through clinical support.
Not every study needs bullet points or complex diagrams. Often, what you need is calibrated control over temperature, agitation, timing, and traceability - plus a diffusion cell you can load and verify at a glance. That is how Raytor approaches How To Test Transdermal Drug Absorption for clinical teams: reduce manual steps, standardize the essentials, and make audits straightforward.
CTA - Bring Clinical-Grade IVRT/IVPT Into Your Workflow
If you're planning a pivotal study or refining a patch or semisolid, talk to Raytor about setting up standards-compliant IVRT/IVPT with synchronized sampling and automated refilling. We'll map your clinical question to a diffusion plan, share ready-to-use method templates, and help you generate permeation data reviewers can trust.
Raytor - manufacturer's precision for clinical confidence.