Pharmaceutical Transdermal Drug Development: Optimizing Formulation Scale-Up
2025-08-21
Pharmaceutical Transdermal Drug Development succeeds when stability and scale-up move in step. At Raytor, we see teams lose time and budget not because the idea is weak, but because in-vitro data, temperature control, and bubble management are inconsistent. This article shares how we approach the pain points - and how our RT806 Vertical Franz Diffusion Cell Tester supports a smoother path from lab batches to reliable production decisions.
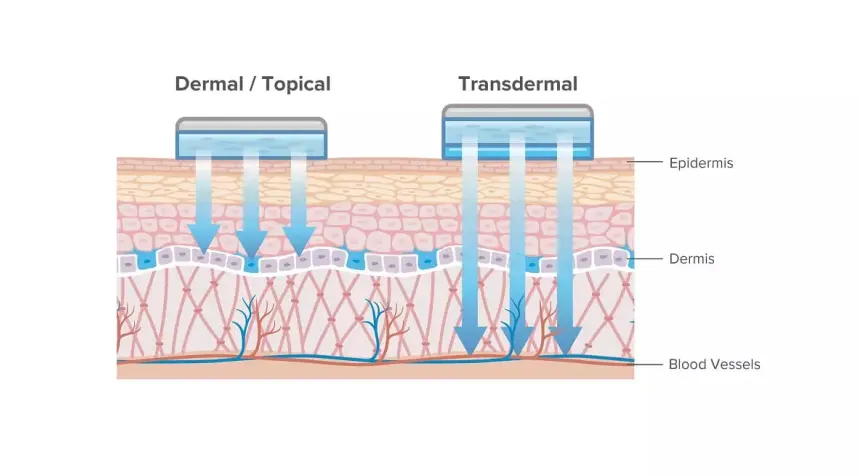
Stability First in Pharmaceutical Transdermal Drug Development
Stability is the backbone of credibility. If your release and permeation data drift, every later decision is shaky. Formulators often face three linked risks: unstable creams or gels, patch irritation from uneven adhesive behavior, and test variance from poor temperature or mixing control. Small issues - like micro-bubbles - can distort diffusion results and force repeats.
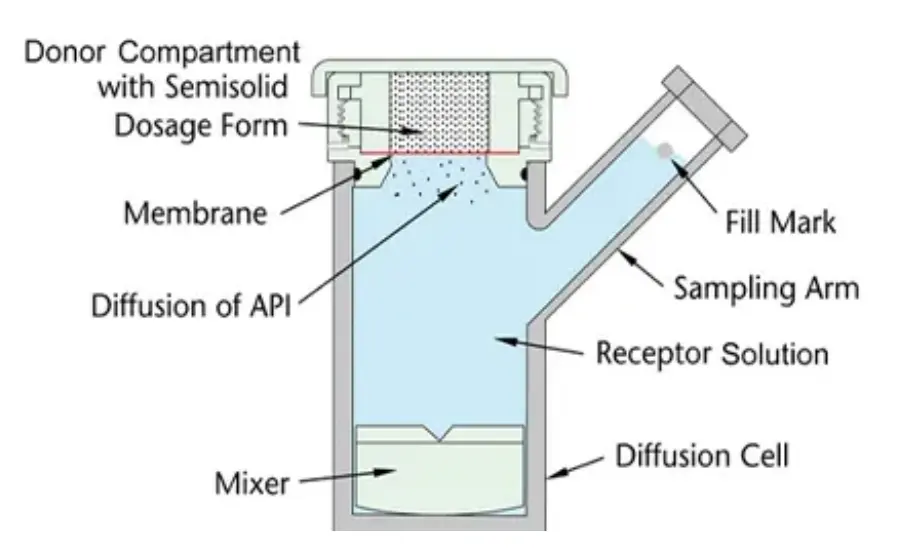
At Raytor, we designed our workflow to keep those factors tight. The RT806 provides heating with a built-in stirrer module and precise control of temperature and speed. It follows USP<1724> specifications for in-vitro release and permeation of semi-solid preparations, including creams, ointments, patches, and gels. The filling-point design helps prevent bubbles, so your baselines stay dependable. When you reduce re-runs, you protect timelines and free your team to focus on formulation insights rather than firefighting.
✅ Built for USP<1724> Compliance
Regulatory alignment is not a “nice to have”; it is an early risk reducer. The RT806 meets the performance requirements outlined in USP<1724> for simulating in-vitro release and permeation procedures. That means your stability study design can map more directly to the expectations reviewers hold, easing method transfer later.
✅ Temperature and Speed Control, Simplified
Stable tests need tight ranges. The RT806 offers room temperature to 55 ℃ with a temperature error ≤ ±0.5 ℃. Stirring covers 200-900 rpm with a speed error < ±10%. Quick adjustment helps you match method conditions fast, cut setup downtime, and keep runs consistent across operators.
Scale-Up Without Surprises: Raytor RT806 in Daily Use
Scale-up fails when lab data cannot be trusted or repeated. Teams tell us their biggest headaches are unplanned method tweaks, operator-to-operator variation, and instruments that are complex to place or clean. We respond with a small-sized, portable layout that is easy to position near your bench or pilot line. Manual sampling keeps operation straightforward and training light.
The RT806 Vertical Franz diffusion cell tester supports practical routines for ointments, creams, patches, and gels. It also fits fast method tuning, so scale-up can start from solid stability evidence rather than hope. For many groups, the ability to prevent bubbles at the filling point is the hidden win - it boosts reproducibility without extra consumables or steps.
✅ Compliant highlights
• Meets USP<1724> performance requirements for in-vitro release and permeation
• Filling-point design helps prevent bubbles for dependable outcomes
• Portable, small-sized system with built-in stirrer and heating
✅ Key specifications (for quick checks)
• Diffusion cell digits: 6
• Sampling method: manual sampling
• Temperature range: room temperature ~ 55 ℃; error ≤ ±0.5 ℃
• Speed range: 200-900 rpm; error < ±10%
A Practical Workflow for Pharmaceutical Transdermal Drug Development
A simple, repeatable workflow helps teams scale with confidence. We advocate a three-step loop that balances speed and rigor.

- Method confirmation
Begin with a short series of in-vitro release tests on RT806 to confirm stirring and temperature settings. The quick-adjust controls make it easy to lock a starting point. Use the filling-point mark to remove bubbles, then inject medium to the mark to help ensure future runs remain bubble-free.
- Stability and robustness
Run permeation studies under small, planned stresses (for example, the edges of your target temperature and speed ranges). The instrument's precise control supports this without complexity. Document any drift and refine the formulation before you commit to larger batches. This step addresses common pain points - adhesive behavior for patches, and microstructural stability for semi-solids.
- Scale-up readiness
Translate your confirmed settings to pilot runs. Because the RT806 is compliant with USP<1724>, your lab methods align with recognized descriptions of diffusion cells. That makes internal reviews faster and reduces back-and-forth during tech transfer.
Not every section needs bullet points; the goal is a smooth reading flow backed by clear checkpoints. Most teams find that fewer repeats, faster setup, and credible data save more time than any single “breakthrough.”
• Why Raytor
Raytor focuses on practical tools that help you do the job well the first time. The RT806 delivers the detailed temperature and speed management needed for reliable transdermal testing, with a layout that fits the bench and a structure that stays simple for daily operation. It is an entry-level transdermal diffusion system - but it meets USP<1724>, supports release and permeation for creams, ointments, patches, and gels, and cuts noise from bubbles by design.
Call to Action
Ready to strengthen stability data and reduce scale-up surprises? Talk to Raytor about the RT806 Vertical Transdermal Diffusion Apparatus, request the Product Brochure, or schedule a quick demo. Let's turn careful in-vitro evidence into faster development progress in Pharmaceutical Transdermal Drug Development.