-
Intelligent Leadership, Precision and Efficiency: Raytor Instruments Successfully Finishes 2025 National Pharmaceutical R&D Innovation Conference
-
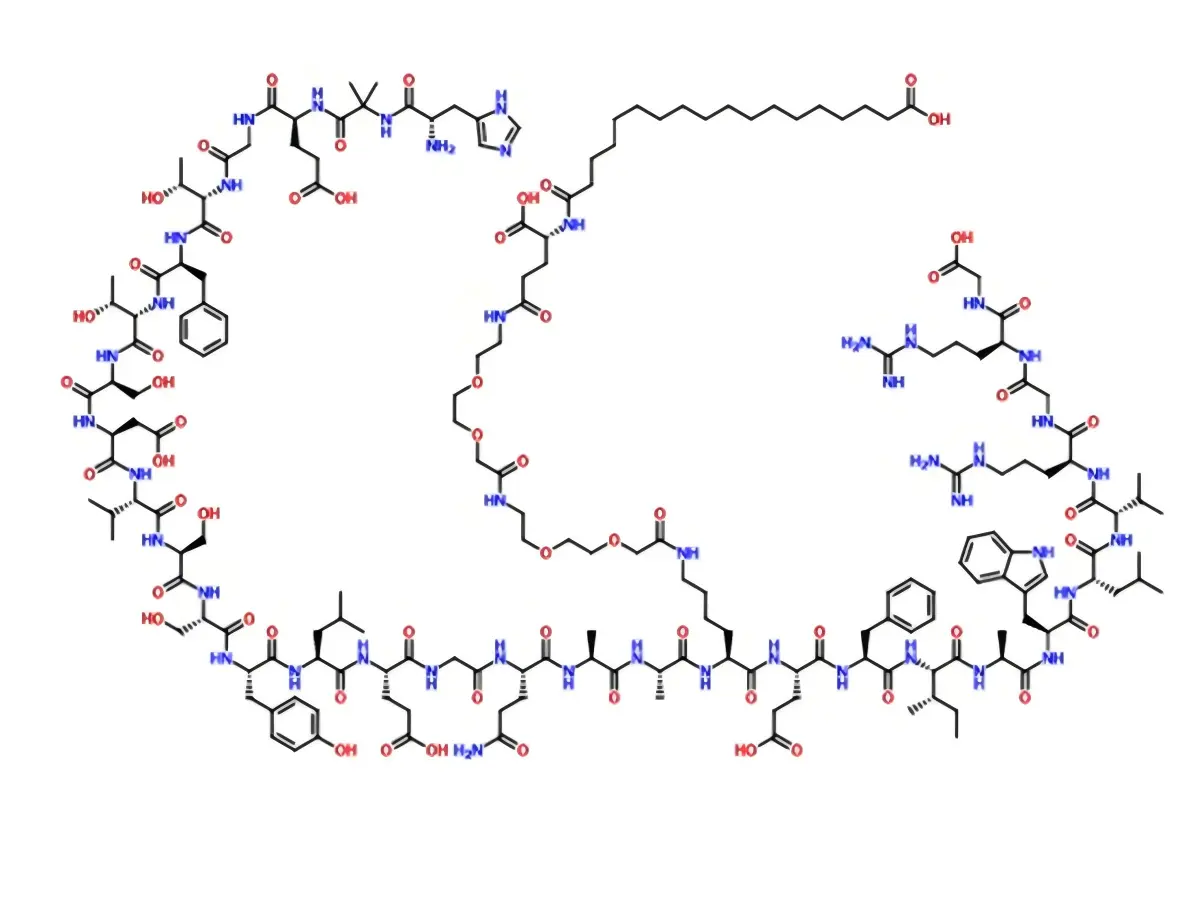
How to increase the absorption of orally administered peptide drugs using Semaglutide as an example?
-
What Is Precision Laboratory Automation and Why It Matters
Choosing the right Laboratory Equipment Supplier can change your results, your timelines, and your audit readiness. Raytor focuses on what most labs struggle with: accuracy, data integrity, and uptime. Since 2015, we've built instruments and support that keep methods consistent and teams moving. Our systems help with dissolution, transdermal studies, and new compound analysis. They fit real workflows, not just specs. Why do leading labs switch to Raytor - and stay? In the next sections, we reveal the standards we meet, the automation behind our speed, and the service model that keeps projects on track.

Built For Real-World Pharmaceutical Work
Raytor is a manufacturer focused on the research, development, production, sales, and service of high-quality laboratory scientific instruments. We have supported pharmaceutical R&D and QC teams from early screening to release testing. Our tools and application support span new compound identification, drug permeability studies, dissolution and solubility evaluation, and transdermal diffusion - covering oral, transdermal, and subcutaneous dosage forms for both pre-formulation and routine quality control.
Depth matters when you are running regulated methods. Our comprehensive R&D platform is led by certified senior pharmaceutical experts who work side-by-side with leading universities and global pharma partners through joint labs. With more than 50 expert researchers and engineers operating across 36,000 m² of facilities, we scale innovation without losing discipline. Lean 6S production management keeps manufacturing tight, while a nationwide sales team and an expanding global partner network provide 24-hour response and local service. The result is predictable lead times, consistent quality, and fast answers when regulators or timelines apply pressure.
Why do labs name Raytor as their Laboratory Equipment Supplier? Because the fundamentals are handled:
- Compliance is designed in - from hardware safeguards to software governance
- Service is integrated - method setup, training, validation support, and after-sales care
- Delivery is fast - backed by controlled processes and responsive technical guidance

Instruments That Remove Bottlenecks
Specifications alone do not protect your schedule. What removes bottlenecks is accuracy you can defend, data you can retrieve, and workflows that remove manual steps. Every Raytor platform is engineered to address three common pain points: calibration drift, data integrity gaps, and time lost to manual handling.
- Dissolution Testing That Meets Global Expectations
Our dissolution solutions are built to comply with Chinese, U.S., and EU Pharmacopoeias and to meet 21 CFR Part 11 requirements. They cover the full USP 1 - USP 4 range, with matching mechanical performance test kits and solvent handling equipment. An intuitive interface shortens training time and makes runs repeatable across operators. A broad accessory library - and rapid customization - lets you match apparatus and methods to product needs without workarounds.
- Method transfer without drama: standardized controls reduce site-to-site variability
- Audit confidence: secure user management and full audit trails support inspections
- Throughput without chaos: automation options minimize wait times and cut manual touches
If you are targeting pharmaceutical dissolution testing solutions, the value is not just the instrument; it is the repeatability and traceability that come with it.
- Transdermal Diffusion With Measurement Discipline
For semi-solids and patches, our manual and automatic diffusion cell systems align with USP requirements and support IVRT/IVPT method development. A compact architecture reduces specimen residue, improving accuracy and repeatability, while end-to-end audit trail functions protect data from sampling to report. Teams evaluating topical formulations gain a clear view of release behavior and performance - without reinventing a workflow each time. This is why many customers search for us using the long-tail phrase transdermal diffusion cell systems.

- New Compound Analysis, Minus The Guesswork
Early R&D requires rapid, defendable insight. Raytor's analytical software uses algorithm-driven detection to characterize drug molecules and explore pharmacokinetic behavior in controlled, simulated environments. Scientists can compare formulations under reproducible conditions, generate fit-for-purpose evidence, and move forward - or stop - based on data, not intuition.
Support, Automation, And Scale That Keep Work Moving
Your lab's limit often isn't instrument count - it's human bandwidth. Raytor's automation and service model address that gap so teams can process more work without stretching headcount.
- Automation That Raises Throughput And Lowers Risk
Multi-batch dissolution platforms run up to 10 batches with precise media dosing and a fully automated cleaning sequence for vessels. Integrated data acquisition, processing, and reporting turn repeated steps into an intelligent workflow. Standardized operations reduce variability, cut transcription errors, and shorten cycle times - ultimately lowering total cost of ownership while increasing experimental confidence.
- Service That Starts Before Installation - And Stays After
Not every barrier is technical. Training, method harmonization, and validation can stall new capability. Raytor's support ecosystem is designed to move teams from "interested" to "operational" quickly:
- Application Experiment Center: collaborative method development and optimization
- Hands-on training & demos: modern facilities for onboarding and skills growth
- Global sales network: local parts, quick logistics, and around-the-clock consultation
- Post-installation care: preventive maintenance, calibration guidance, and compliance support
We continue to expand demonstration and training centers so customers can test workflows, collect evidence for internal stakeholders, and prepare for regulatory reviews with confidence. From the first method draft to commercial scale, Raytor stays accountable as your Laboratory Equipment Supplier.
A Practical Path Forward
If your team is pushing to reduce deviations, accelerate release, or scale studies without expanding headcount, it may be time to reassess your Laboratory Equipment Supplier. Raytor combines compliant design, automation, and responsive service to deliver results that hold up - on the bench and in the audit room.
Call To Action: Ready to improve accuracy, compress timelines, and simplify inspections? Contact Raytor for a tailored consultation, application notes, or a live demonstration. See how our platforms and support model translate into faster methods, clearer data, and fewer roadblocks for your pipeline.