The Critical Step in Formulation Optimization: How Different Excipient Ratios Influence Drug Dissolution and Permeation Fate
2025-12-15

Background
Cyclodextrin, as a common pharmaceutical excipient, possesses a unique molecular structure—shaped like a "hollow cylinder" with a hydrophobic inner wall and a hydrophilic outer wall. This "outward-friendly and inward-distant" characteristic enables it to encapsulate poorly soluble drug molecules in the cavity, forming inclusion complexes, thereby significantly enhancing the solubility and stability of the drug and reducing its own irritability. In practical applications, choosing the right cyclodextrin is only the first step. The proportion of excipients is equally crucial. Only with an appropriate ratio can the drug achieve an ideal state between dissolution and penetration, truly exerting the expected therapeutic effect of the prescription.
Case
The blood drug concentration of a drug in the body depends on its dissolution and osmotic absorption in the small intestine. In this case, API is an alkaline BCSⅡ class drug, which will precipitate in the small intestine of the human body. Through the excipient screening experiment, it has been found that a suitable excipient – cyclodextrin, which can enhance the in vitro dissolution effect of the drug. However, the proportion of the excipient to be mixed with API to achieve the ideal state between dissolution and penetration of API requires further experimental screening.

Experimental Design
This experiment utilized the penetration model excipient &API compatibility penetration (PAMPA method) study in Raytor NCE DP to conduct a comparative study on the compatibility of 1:1 and 1:2 (API : excipients). The experimental conditions are as follows:
| Condition | Control | 1:2 β-Cyclodextrin | 1:1 β-Cyclodextrin |
| Donor Side | pH 6.5 Buffer | pH 6.5 Buffer | pH 6.5 Buffer |
| Acceptor Side | pH 7.4 Buffer | pH 7.4 Buffer | pH 7.4 Buffer |
| Sample | API | API | API |
| Excipient | None added | According to the 1:2 ratio design, β-cyclodextrin was injected into the system at 20min after the start of the experiment | According to the 1:1 ratio design, β-cyclodextrin was injected into the system at 20min after the start of the experiment |
Experimental Results
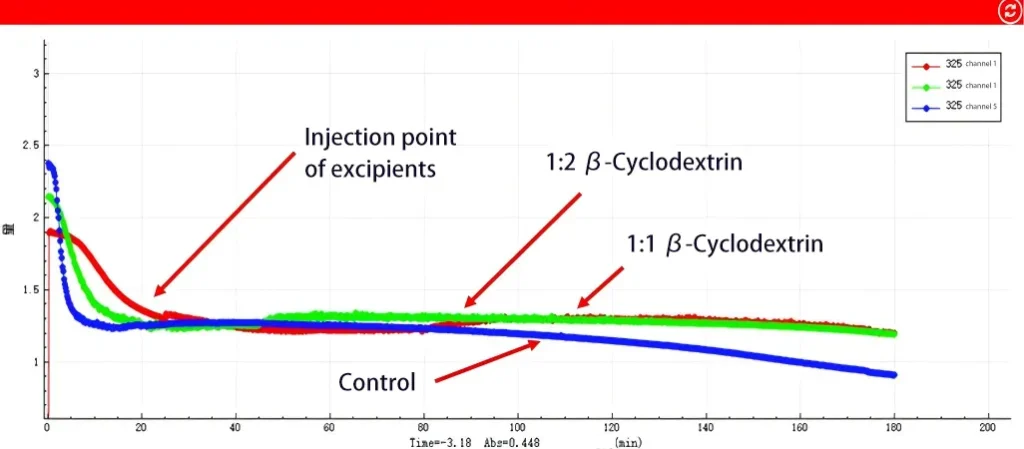
Changes in drug concentration at the donor side
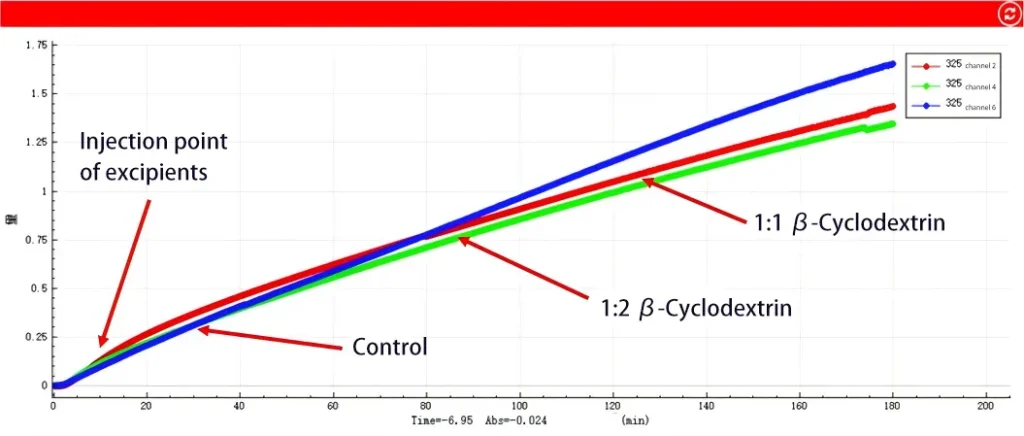
Changes in drug concentration at the acceptor side
Conclusions:
Donor Side (Dissolution Behavior):
The API (blue line) will gradually precipitate. After adding β -cyclodextrin for 20 minutes, the concentration of API no longer decreased compared with the control, indicating that β -cyclodextrin can inhibit drug precipitation. This result is consistent with the in vitro dissolution experiment conducted by the customer. Moreover, regardless of whether the ratio is 1:1 (red line) or 1:2 (green line), the drug concentration is stably maintained and no longer decreases. This proves that cyclodextrin indeed effectively inhibits drug precipitation, and the improvement effects on solubility under the two ratios are similar.
Acceptor Side (penetration behavior) :
The concentration of API (blue line) increased normally, and its effective permeability (Peff) value was -3.28, which belongs to BCSⅡ class drugs, consistent with the biologics classification of API. However, after the addition of β -cyclodextrin, the effective permeability of the drug decreased significantly. Moreover, the inhibitory effect of a 1:2 ratio (green line) on osmosis is stronger than that of a 1:1 ratio (red line).
This indicates that the addition of β -cyclodextrin can indeed effectively enhance the solubility of API, and its effect of enhancing dissolution is similar under different ratios (1:1 and 1:2). However, in terms of osmotic absorption, β -cyclodextrin shows a significant inhibitory effect, and this inhibitory effect intensifies with the increase of its addition ratio. That is, the more excipients are added, the more significant the decline in drug osmotic absorption becomes.
Discussion
This suggests that auxiliary materials are a "double-edged sword". Blindly pursuing the solubility index and increasing the amount of excipients may come at the expense of bioavailability. It warns us that the screening and dosage optimization of excipients must be based on a systematic assessment of the comprehensive effect of "dissolution-penetration". The PAMPA model provided by Raytor NCE DP precisely helps us predict risks and make scientific decisions at an early stage.



