Gel 、Microemulsion (The release of active ingredients)

Gel is a thick liquid or semi-solid preparation with gel characteristics made from a raw drug and excipients that can form a gel; and microemulsion, as a new type of dosage form, utilizes surfactants to enable the oil and water phases in the preparation to exist harmoniously, so that the active ingredient stays in the aqueous phase or the oil phase.
Get Free Quote
Experimental parameters
| Test system | RT800 Automated Transdermal Diffusion System |
| Diffusion cell | A standard Franz vertical diffusion cell with an inner aperture of 15mm |
| Sample loading volume | approximately 1 mL |
| Temperature | 37℃± 0.5℃ |
| Sampling time | 0.25、0.5、1、2、3、4 hours |
| Detector | UV 265nm |
| Flow rate | 1.0 mL/min |
| Mobile phase | Acetonitrile - sodium dihydrogen phosphate solution |
| Chromatographic column | Intersil ODS-3 5μm |
Experimental results
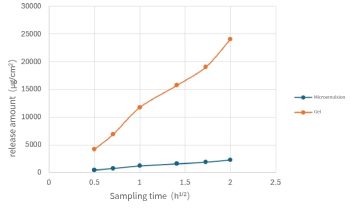
Figure 1. Investigation on the differences in drug release caused by different dosage forms The release rate of the drug in the sample is expressed by the cumulative drug release amount (μg/cm ²) and the square root of time (t) (i.e., Higuch's formula: M=kt1/2), where the k value represents the drug release rate. The results are shown in Figure 1. The release amount of active ingredient was different in different dosage forms, and the gel formulation group had a higher release amount in all sampling points. In addition to the Dosage of drug release, the drug release rate can also reflect the effect of different preparations on the release of the same active ingredient. The drug release rate of the gel is 12711, and that of the microemulsion is 1139.5
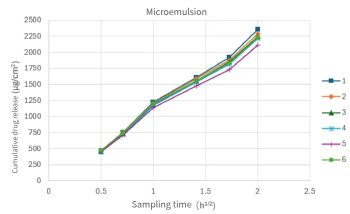
Figure 2. Repeatability test To ensure that the test results accurately reflect the differences among samples, it is necessary to examine the test conditions. The relevant guidelines for the validation of analytical methods in the ChP state that the purpose of the validation of analytical methods is to prove that the established method is suitable for the corresponding testing requirements. The key indicator for verification is repeatability.
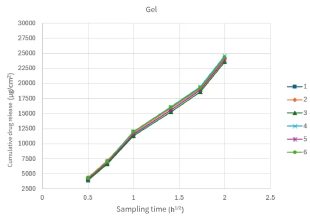
Figure 3. The cumulative release curves of the two experiments have different coincidence degrees under the six parallel experiments. The relative standard deviations of the cumulative drug release at the sampling endpoint were 3.52% and 1.5% respectively. It can be seen that different preparations have different abilities to maintain good repeatability in experiments.
Conclusion:
From the above case, it can be seen that the automatic sampling transdermal diffusion system can provide strong and favorable support for the in vitro release research of drugs. Moreover, under reasonable and appropriate release conditions, it can compare the cumulative drug release amount and cumulative drug release rate among different dosage forms of the same active ingredient, significantly differentiating the differences among different dosage forms.

