Drug Permeation Studies explore how a drug moves from a formulation, across the skin, and into the body. They turn the skin from a simple "shield” into a measurable scientific system. By tracking how fast and how far active ingredients travel, these studies help scientists see whether a cream, gel, ointment, or patch really works as intended. They also expose how unpredictable real skin can be. Why do some products show strong effects on one person but almost none on another? And what hidden barriers inside the skin are responsible?

Why the Skin Barrier Deserves So Much Attention
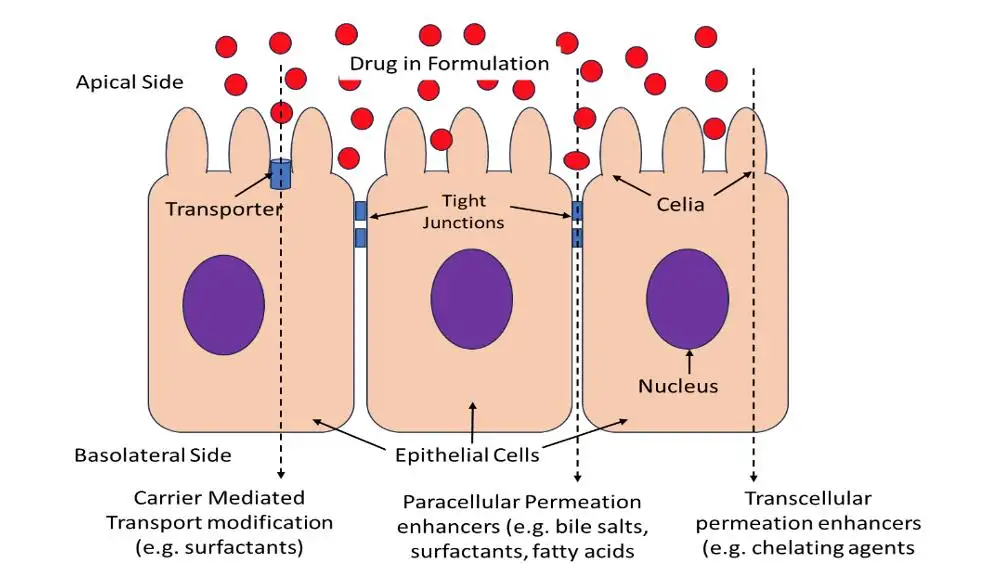
On paper, a topical or transdermal formulation should deliver a defined amount of active across the skin at a predictable rate. In practice, the skin barrier is heterogeneous, dynamic, and highly individual. Drug Permeation Studies help turn that biological complexity into numbers that formulation, QA, and regulatory teams can trust.
Several day-to-day challenges all trace back to the skin barrier:
• Unpredictable penetration: the same cream may behave differently on dry versus oily skin, or on the forearm versus the back.
• Regulatory expectations: agencies increasingly expect in vitro release and permeation data that support label claims and align with standards such as USP <1724>.
• Expensive late-stage failures: if permeation properties are poorly understood, problems may appear only in clinical testing or after launch.
By mapping how quickly and how far an active migrates from the dosage form, across the skin, and into a receptor medium, Drug Permeation Studies transform these unknowns into measurable performance. Typical questions they answer include:
• Does a patch release the active at a stable rate over several hours?
• Does a gel really move beyond the stratum corneum?
• What happens to permeation when the formulation, excipient level, or manufacturing process changes?
The earlier such questions are addressed, the easier it is to shorten development cycles, compare prototypes, and remove weak candidates.
(Bioavailability Enhancement Service & Permeability Solutions | Vici Health Sciences)
What Drug Permeation Studies Look Like in the Lab
In real laboratory workflows, Drug Permeation Studies are most commonly run on vertical Franz diffusion cells. Such systems allow researchers to recreate release and permeation through a skin or membrane barrier in a controlled in vitro environment, removing many of the hurdles associated with in vivo experiments.
A well-designed vertical diffusion cell tester usually provides:
• Stable temperature conditions - even slight temperature drifts can influence how quickly actives permeate.
• Controlled mixing - stable stirring conditions reduce concentration gradients in the receptor phase and improve data reliability.
• Dedicated bubble control - surface bubbles cut down the active diffusion area and may result in underestimation of permeation.
A seemingly minor but very practical design element is the filling point on the diffusion cell. When the receptor medium is carefully degassed, bubbles are removed, and the cell is filled exactly to this mark, the risk of new bubbles forming during the experiment drops sharply. The result is cleaner permeation profiles and fewer unexplained outliers.
With an appropriate Franz cell configuration and a compliant protocol, Drug Permeation Studies can be applied across a wide range of semi-solid preparations, such as:
• Dermatological creams and lotions
• Ointments used for wound care or barrier protection
• Topical gels designed for rapid local delivery
• Transdermal systems intended for systemic drug delivery
Instead of relying only on theoretical release models, you see how the product behaves under standardized, repeatable conditions.

Selecting a Diffusion Cell System that Works Every Day
Once a team recognizes how valuable Drug Permeation Studies can be, a new question appears: how do you make these tests part of the routine, rather than an occasional specialist exercise?
Many laboratories still rely on diffusion systems that are oversized, complex to run, and daunting for new staff. Typical issues include:
• Long training periods before analysts can operate the system independently
• Setup mistakes that quietly add variability to permeation profiles
• Bulky instruments that take up valuable bench space while remaining underutilized
A compact vertical Franz diffusion cell tester, built as an entry-level transdermal diffusion platform, helps eliminate these barriers. Its small footprint and straightforward manual operation make it easy to relocate and plug into routine workflows. At the same time, it can still satisfy the performance and design criteria for in vitro release and permeation testing set out in USP <1724>.
Typical strengths include:
• Intuitive, minimal design - quick to learn, easy to clean and maintain, ideal for training labs and everyday QC.
• A filling point design that discourages bubble formation - more reliable Drug Permeation Studies and fewer misleading outliers.
• Conformance with Franz diffusion cell descriptions in USP <1724> - smoother reporting for audits, validations, and regulatory filings.
• With this mix of regulatory-ready design and user-friendly operation, the system helps make Drug Permeation Studies a standard lab tool instead of a specialist exercise.
From Better Data to Better Products
Once Drug Permeation Studies become easy to perform and interpret, the benefits spread across the pipeline. Formulators see sooner how skin barriers react to formulation tweaks. QA and QC teams can verify batch-to-batch performance using permeation profiles. CROs can offer clients data sets that are both consistent and defensible.
Ultimately, the outcome is the same: more trustworthy data, clearer understanding of skin barrier behavior, and a smoother path from early R&D to regulatory approval and market launch.
If your team develops or tests creams, ointments, gels, or patches and you want a more reliable view of how they cross the skin barrier, now is a good moment to revisit your Drug Permeation Studies setup. The right compact vertical Franz diffusion cell system can streamline in vitro release and permeation testing - and help replace guesswork in transdermal development with evidence you can stand behind.



