
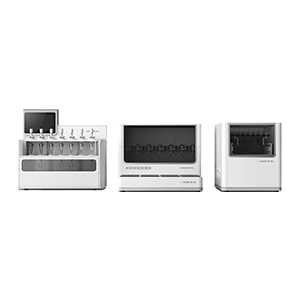
Flow-Through Cell Dissolution System
Flow-Through Cell Dissolution System works best with poorly soluble drugs as well as extended-release formulations. This system fulfills the requirements of different audit standards including Chinese, U.S. and EU Pharmacopoeia and 21 CFR Part 11. The system supports USP 4 dissolution testing by offering mechanical performance kits together with solvent handling equipment. Dissolution studies benefit crucially from a user-friendly interface that produces accurate results at high-efficiency rates. This technology can be adjusted through customized accessories and quick variability so laboratories can perform diverse task requirements.
Get Free Quote
Explore Products
Talk to Our Engineer Now
Flow-Through Cell Dissolution System
RT700 Flow-through Cell Dissolution System
- Patented Filter Components
- Immersion Thermostatic Water Baths
- 0/120Hz Dual-pulse Mode
- Compatible with Both Open and Closed-loop Modes

RT710 USP Apparatus 4 Flow-through Dissolution System
- Velocity Range:1~40mL/min
- Sampling Volume Error:≤± 2% (closed-loop mode), ≤± 5% (open-loop mode)
- Pump Type: syringe pump
- Number of Channels: 7
Features
The system meets the regulatory standards of Chinese Pharmacopoeia together with those from U.S. Pharmacopoeia and EU Pharmacopoeia which guarantees pharmaceutical quality control objectives. Compliance with 21 CFR Part 11 guarantees secure, traceable data management.
Multi-channel Synchronized Testing
Supports simultaneous dissolution testing of multiple samples, improving efficiency.
Automated Temperature Control System
Automatically adjusts temperature, ensuring consistent testing conditions.
High-Precision Data Acquisition
High-precision data acquisition involves collecting and analyzing data accurately and in real time
Intelligent Analysis Software
Provides data acquisition, comparison, and visualization, simplifying analysis.
Compliant with GMP and GLP Standards
Meets pharmaceutical industry compliance requirements, ensuring data reliability.
Complies with 21 CFR Part 11
meets audit trails to ensure data security
Resources

12 Positions Automated Dissolution tester Leaflet
Download

Flow-through cell dissolution system Leaflet
Download

RT612-ST Automatic Sampling General Dissolution System
Cases
This USP 4 dissolution testing system allows accurate drug release analysis through its capabilities to regulate media flow rates. The testing system works best for drugs that show low solubility and extended-release medications.






Have A Business Inquiry?
Interested to find out more about how our products and services can serve your business needs? Drop us an online inquiry and our qualified professionals will reach out to you.
Get Free Quote
The system features a built-in interface that makes testing processes simpler while minimizing operational mistakes. The system increases both operational speed and data accuracy because of automated solvent management and data capture capabilities.
Multiple flow-through dissolution accessories from the range allow users to create various testing setups. Rapid customization services enable pharmaceutical research laboratories to meet their wide range of quality control requirements.

RT600-ST.png)
RT612-ST.png)
RT600.png)
