News
Stay informed with Dissolution Tester News, featuring the latest advancements, industry trends, and updates in pharmaceutical testing technology. Discover new features, regulatory changes, and innovative solutions for accurate drug release analysis, ensuring quality and compliance in drug development and manufacturing.
Filter by Popular Tags

A Journey into Intelligent Manufacturing!
We were thrilled to welcome our partners to the Raytor Instruments headquarters! It was an incredible day filled with insightful discussions, hands-on demonstrations of our latest lab equipment, and a ...
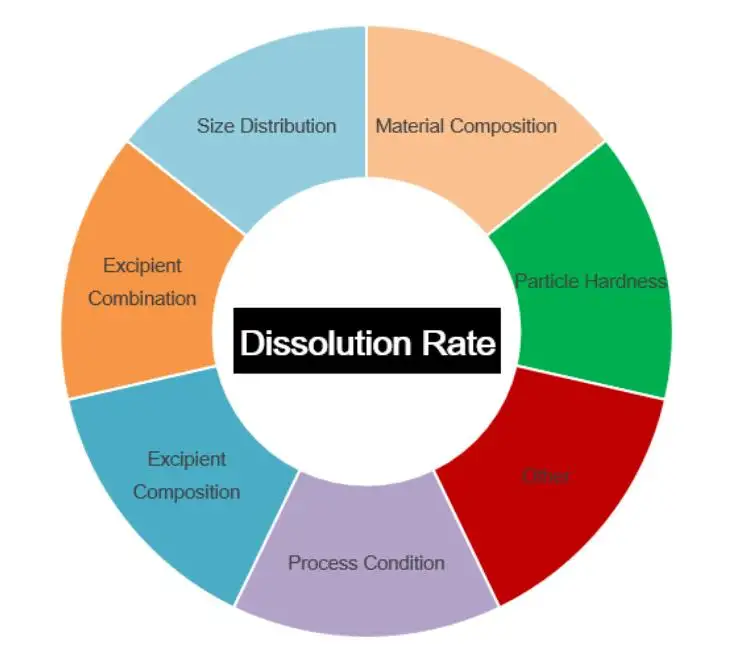
Drug Dissolution Rate Analysis: Why It Drives Oral Product Success
Drug Dissolution Rate Analysis sits at the center of oral drug development because it connects what you make in the lab to what patients experience in the clinic. When an ...

Pharmaceutical Solubility Testing: Why It Matters And What It Really Measures
Solubility is one of the earliest and most consequential gates a compound passes through on its way to becoming a medicine. It shapes exposure, variability, and in many cases whether ...

Automated Laboratory Testing Solutions Guide: 2026 Multi-Batch Dissolution
The ground is shifting for QC and R&D labs in 2026. Sample volumes are rising, release windows are tightening, and dissolution testing must keep pace without sacrificing data integrity. Multi-batch ...
Physical Properties of Pharmaceuticals Explained for QC and Stability
Physical Properties of Pharmaceuticals shape how dosage forms perform from the factory to the pharmacy. This guide will help you master the following: why these properties drive QC and stability, ...

Transdermal Diffusion Testing: A 2026 Field Guide for Reliable IVRT/IVPT
Transdermal Diffusion Testing is rapidly becoming the backbone of how labs evaluate patches and semisolid drug products. In 2026, it's not enough to "get a curve." Teams need release and permeation ...
